Efficacy and Safety of Elamipretide in Individuals With Primary Mitochondrial Myopathy: The MMPOWER-3 Randomized Clinical Trial
- PMID: 37268435
- PMCID: PMC10382259
- DOI: 10.1212/WNL.0000000000207402
Efficacy and Safety of Elamipretide in Individuals With Primary Mitochondrial Myopathy: The MMPOWER-3 Randomized Clinical Trial
Abstract
Background and objectives: Primary mitochondrial myopathies (PMMs) encompass a group of genetic disorders that impair mitochondrial oxidative phosphorylation, adversely affecting physical function, exercise capacity, and quality of life (QoL). Current PMM standards of care address symptoms, with limited clinical impact, constituting a significant therapeutic unmet need. We present data from MMPOWER-3, a pivotal, phase-3, randomized, double-blind, placebo-controlled clinical trial that evaluated the efficacy and safety of elamipretide in participants with genetically confirmed PMM.
Methods: After screening, eligible participants were randomized 1:1 to receive either 24 weeks of elamipretide at a dose of 40 mg/d or placebo subcutaneously. Primary efficacy endpoints included change from baseline to week 24 on the distance walked on the 6-minute walk test (6MWT) and total fatigue on the Primary Mitochondrial Myopathy Symptom Assessment (PMMSA). Secondary endpoints included most bothersome symptom score on the PMMSA, NeuroQoL Fatigue Short-Form scores, and the patient global impression and clinician global impression of PMM symptoms.
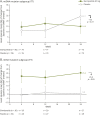
Results: Participants (N = 218) were randomized (n = 109 elamipretide; n = 109 placebo). The m0ean age was 45.6 years (64% women; 94% White). Most of the participants (n = 162 [74%]) had mitochondrial DNA (mtDNA) alteration, with the remainder having nuclear DNA (nDNA) defects. At screening, the most frequent bothersome PMM symptom on the PMMSA was tiredness during activities (28.9%). At baseline, the mean distance walked on the 6MWT was 336.7 ± 81.2 meters, the mean score for total fatigue on the PMMSA was 10.6 ± 2.5, and the mean T score for the Neuro-QoL Fatigue Short-Form was 54.7 ± 7.5. The study did not meet its primary endpoints assessing changes in the 6MWT and PMMSA total fatigue score (TFS). Between the participants receiving elamipretide and those receiving placebo, the difference in the least squares mean (SE) from baseline to week 24 on distance walked on the 6MWT was -3.2 (95% CI -18.7 to 12.3; p = 0.69) meters, and on the PMMSA, the total fatigue score was -0.07 (95% CI -0.10 to 0.26; p = 0.37). Elamipretide treatment was well-tolerated with most adverse events being mild to moderate in severity.
Discussion: Subcutaneous elamipretide treatment did not improve outcomes in the 6MWT and PMMSA TFS in patients with PMM. However, this phase-3 study demonstrated that subcutaneous elamipretide is well-tolerated.
Trial registration information: Trial registered with clinicaltrials.gov, Clinical Trials Identifier: NCT03323749; submitted on October 12, 2017; first patient enrolled October 9, 2017.
Clinicaltrials: gov/ct2/show/NCT03323749?term = elamipretide&draw = 2&rank = 9.
Classification of evidence: This study provides Class I evidence that elamipretide does not improve the 6MWT or fatigue at 24 weeks compared with placebo in patients with primary mitochondrial myopathy.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
A. Karaa: received research grant, reimbursement for travel, and consulting payments from Stealth BT, Sanofi Genzyme, and Takeda; received research grant and reimbursement for travel from Protalix and REATA; received research grants from Astellas, MitoBridge, Cyclerion, PTC therapeutics, and Idorsia; received consulting payments from MitoBridge, Astellas, Alexion, Lumleian, Homology, MitoBridge, Akros, Neurovive, Reneo, and Zogenix; and is on the medical advisory board of MitoAction and on the scientific and medical advisory board of the United Mitochondrial Disease Foundation; is a founder and board member of the mitochondrial care network, President of the Mitochondrial Medicine Society; and is an investigator in the North American Mitochondrial Disease Consortium. E. Bertini: received funds for consulting and Advisory Board from PTC, Roche, Novartis, Biogen, and Sarepta and is responsible for an HCP that is part of both the European Rare Neurologic Disorders (ERN RND) and the ERN-NMD (neuromuscular disorders) network. V. Carelli: received research grant from Stealth BioTherapeutics; consulting payments for Advisory Board and speaker honoraria from Chiesi Farmaceutici; clinical trial funding from Stealth BioTherapeutics, Santhera Pharmaceuticals, and GenSight Biologics; research grants from the Italian Ministry of Health, Italian Ministry of University and Research, Telethon-Italy, and European Union (Horizon 2020 program); and research funding from patient donations and serves the scientific advisory board of IFOND and MITOCON. G. M. Enns: Consulting—Evvia Therapeutics/cofounder, AllStripes/consultant, Glycomine/consultant, Hemoshear/consultant, Homology Medicines/consultant, Moderna Therapeutics/consultant, M6P Therapeutics/consultant, Ultragenyx/consultant. DMC member—Amicus Therapeutics, Audentes Therapeutics, Biomarin, Modis Therapeutics, Paradigm Biopharmaceuticals, Passage Bio, RegenxBio. Clinical Trials (site PI)—Aeglea Biotherapeutics, Artcurus Therapeutics, Astellas Pharma, Biomarin, LogicBio, Moderna, PTC Therapeutics, Stealth BioTherapeutics, and University of Florida. M.J. Falk: Marni J. Falk, MD, is engaged with several companies involved in mitochondrial disease therapeutic preclinical and/or clinical stage development not directly related to the work, including as an advisory board member with equity interest in RiboNova, Inc., as a scientific board member as a paid consultant with Khondrion and Larimar Therapeutics, as a paid consultant (Astellas [formerly Mitobridge] Pharma Inc., Casma Therapeutics, Cyclerion Therapeutics, Epirium Bio, HealthCap VII Advisor AB, Imel Therapeutics, Minovia Therapeutics, NeuroVive Pharmaceutical AB, Reneo Therapeutics, Stealth BioTherapeutics, and Zogenix Inc.), and/or as a sponsored research collaborator (AADI Bioscience, Astellas Pharma Inc., Cyclerion Therapeutics, Epirium Bio (formerly Cardero Therapeutics), Imel Therapeutics, Khondrion, Minovia Therapeutics Inc., Mission Therapeutics, NeuroVive Pharmaceutical AB, PTC Therapeutics, Raptor Therapeutics, REATA Inc., Reneo Therapeutics, RiboNova Inc., Standigm Inc., and Stealth BioTherapeutics). MJF also receives royalties from Elsevier, speaker fees from Agios Pharmaceuticals, and educational honorarium from PlatformQ. G. Gorman: received research grant, consulting payments for Advisory Board, and clinical trial funding from Stealth BioTherapeutics. R. Haas: received research grant, reimbursement for travel, and consulting payments from Stealth BT; is on the scientific and medical advisory board of the United Mitochondrial Disease Foundation and the advisory board for MitoBridge; received clinical trial funding from Edison Pharmaceuticals, Stealth BioTherapeutics, Horizon Pharma (previously Raptor), Reneo Pharmaceuticals, PTC Therapeutics, Acadia Pharmaceuticals, and Sarepta Therapeutics; and received grant funding through the FDA Orphan Products Clinical Trials Grants Program (previously Orphan Products Grants; #1RO1FD004147) and the NIH (U54 NS078059). M. Hirano: has received research support, honoraria, or both from Stealth BioTherapeutics, Entrada Therapeutics, and Modis Therapeutics (wholly owned subsidiary of Zogenix) and grant support from the NIH (U54 NS078059 and P01 HD32062), Department of Defense (FPA W81XWH2010807), Muscular Dystrophy Association (577392), and J. Willard and Alice S. Marriott Foundation. Columbia University has a patent for deoxynucleoside therapies for mitochondrial DNA depletion syndrome including TK2 deficiency, which is licensed to Modis Therapeutics, a wholly owned subsidiary of Zogenix Inc.; this relationship is monitored by an unconflicted external academic researcher. Dr. Hirano is a coinventor of this patent. CUIMC has received royalty payments related to the development and commercialization of the technology; Dr. Hirano has received shares of the royalty payments following Columbia University policies. He is on the scientific and medical advisory board of the United Mitochondrial Disease Foundation and Barth Syndrome Foundation and the Research Advisory Committee of the Muscular Dystrophy Association. T. Klopstock: received clinical trial funding from Stealth BioTherapeutics, Santhera Pharmaceuticals, Khondrion, and GenSight Biologics; received reimbursement for travel and consulting payments from Santhera Pharmaceuticals, GenSight Biologics, and Chiesi GmbH; and is the Speaker of the German network for mitochondrial disorders (mitoNET). Thomas Klopstock is supported by the German Federal Ministry of Education and Research (BMBF, Bonn, Germany) through grants to the German Network for Mitochondrial Disorders (mitoNET, 01 GM1906A) and to the E-Rare project GENOMIT (01 GM1920B). M. K. Koenig: receives research/grant support from Stealth BT, EryDel S.p.A., Ultragenyx Pharmaceuticals, BioElectron Technology Corporation/PTC Therapeutics, NIH, People Against Leigh Syndrome, Marinus Pharmaceuticals, TEVA Pharmaceuticals, Esai Pharmaceuticals, and Reneo Pharmaceuticals; serves on speaker's bureau/advisory board for Novartis Pharmaceuticals, Greenwich Pharmaceuticals, Stealth BT, Taysha Gene Therapies, and Modis Therapeutics; serves on scientific and medical advisory board for the Mitochondrial Medicine Society, Tuberous Sclerosis Complex Alliance, TANGO2 Research Foundation, and People Against Leigh Syndrome; coinventor of “Topical Rapamycin Therapy” licensed to LAM Therapeutics. C. Kornblum: has received travel funding and/or speaker honoraria from Sanofi Genzyme, Novartis, Santhera, and Fulcrum Therapeutics, acknowledges financial support as advisory board member and/or primary investigator for Stealth BioTherapeutics, Inc., Sanofi Genzyme, Amicus Therapeutics, Roche Pharma AG, Hormosan, Fulcrum Therapeutics, receives research support from the German Federal Ministry of Education and Research (BMBF), and is a member of the European Reference Network for neuromuscular diseases (EURO-NMD) and cocoordinator of mitoNET (BMBF funded). C. Lamperti: received research grant from and is supported by the Telethon (GSP16001) and by the E-Rare project GENOMIT (01 GM1920B), Italian Ministry of health RF: 2016–02361495, and Chiesi faramceuticis, member of the European Reference Network for neuromuscular diseases (EURO-NMD. A. Lehman: has received research grants, reimbursement for travel, or consulting payments from Shire-Takeda, Sanofi-Genzyme, Ultragenyx, Horizon Pharma, and Amicus. N. Longo: has received speaker honoraria from Alnylam, Amicus Therapeutics, ACI Clinical trials, BioMarin, BridgeBio/CoA Ther, Censa/PTC Ther., Chiesi/Protalix, CTI-Clinical Trial, Genzyme/Sanofi, Hemoshear, Horizon Pharma, Jaguar Gene Therapy, Leadiant Biosciences, Moderna, Nestle’ Pharma, Recordati, Reneo, Shire/Takeda, Synlogic, and Ultragenix; also receives clinical trial support from Aeglea, Amicus Therapeutics, Audentes/Astellas, AvroBio, BioMarin, Censa/PTC Ther., Chiesi/Protalix, Genzyme/Sanofi, Hemoshear, Homology, Horizon Pharma, Moderna, Nestle’ Pharma, Pfizer, Reneo, Retrophin, Shire/Takeda, Stealth BioTherapeutics, Synlogic, and Ultragenix. M. Judit Molnar: received research grant, reimbursement for travel, and consulting payments from Stealth BT, Sanofi Genzyme, Biogen, Amicus Therapeutics, PTC Therapeutics, Novartis Pharma, Takeda, and Richter Gedeon Plc. Molnar MJ is supported by the National Brain Foundation 2.0. H.Phan: received research grant, reimbursement for travel, and consulting payments from Stealth BT, Shire, Sarepta, Fibrogen, Pfizer, Ovid, Emalex, GeneTx, Genetech, Applied Therapeutics, Teva, Italofarmaco, and Eisai. S. Parikh: has no relevant disclosures to report R. D.S. Pitceathly: received reimbursement for travel and consulting payments from Stealth BioTherapeutics; a research grant, reimbursement for travel, and consulting payments from Reneo Pharmaceuticals; and consulting payments from Abliva. F. Scaglia: has received research grants from Stealth BT, Reata Pharmaceuticals, PTC Therapeutics (Edison Pharma), Horizon Pharma (Raptor Pharma), Entrada Therapeutics, Astellas Pharma, Modis Therapeutics, and the NIH (U54 NS078059). He has received research support from the Mervar Foundation and the Courage for a Cure Foundation. He is on the Board of the Mitochondrial Medicine Society and is an investigator for the North American Mitochondrial Disease Consortium and the Frontiers in Congenital Disorders of Glycosylation Consortium. S. Servidei: responsible for an HCP that is part of the European Rare Neurologic (ERN) NMD network. R. P. Saneto: received research grant and reimbursement for travel and consulting payments from Stealth Bio Therapeutics, received clinical trial funding from Edison Pharmaceuticals, and served on the DSMB board for REATA Pharmaceuticals. M. Tarnopolsky: has received speaker honoraria from Sanofi-Genzyme. He is the President and CEO of Exerkine Corporation that produces nutraceuticals that target mitochondrial dysfunction in obesity and aging. A. Toscano: has received reimbursement for educational activities from Sanofi Genzyme and Amicus and honorarium as component of Sanofi Genzyme Pompe Disease European Board. J. Van Hove: has received reimbursement for travel and consulting payments from Stealth BT. J. Vissing: has received research and/or travel support and/or speaker honoraria from UCB Pharma, Edgewise Therapeutics, Sanofi/Genzyme, Fulcrum Therapeutics, Argenx, Biogen, Lupin Limited, and Alexion Pharmaceuticals and served on advisory boards or as a consultant for Argenx, Roche, Viela Bio, Biogen, Amicus Therapeutics, Genethon, PTC Therapeutics, Sanofi/Genzyme, Ultragenyx Pharmaceutical, Fulcrum Therapeutics, ML Bio Solutions, Sarepta Therapeutics, Novartis Pharma AG, Stealth Biotherapeutics, Zogenix, Regeneron, UCB Pharma, Viela Bio, Lundbeck Pharma, and Lupin Limited. J. Vockley: received research grant, reimbursement for travel, and consulting payments from Stealth BioTherapeutics. M. Mancuso: received research grant, reimbursement for travel, and consulting payments from Stealth BT, Shire, Sanofi Genzyme, Khondrion, Abliva, Reneo, and Zogenix. Mancuso is supported by the Telethon (GSP16001) and by the E-Rare project GENOMIT (01 GM1920B). Mancuso is part of the European Rare Neurologic (ERN) NMD network. B. H. Cohen: received research grant, reimbursement for travel, and consulting payments from Stealth BT; received research grants from Reata pharmaceuticals, BioElectron Technology (Edison Pharma), and Horizon Pharma (Raptor Pharma); received travel support from Reata; serves on the UMDF Board of Trustees; and is an investigator for the NAMDC. D. A. Brown is an employee of Stealth BioTherapeutics and receives compensation and benefits commensurate with full-time employment. J. Finman and James Schiffer are consultants for Stealth Biotherapeutics and receive compensation commensurate with their consulting activities. Go to
Figures


References
-
- Mancuso M, Hirano M. NORD physician guide to mitochondrial myopathies (MM). Nat Org Rare Disord. 2016:1-8.
-
- Rahman J, Rahman S. Mitochondrial medicine in the omics era. Lancet. 2018;391(10139):2560-2574. - PubMed
-
- Tarnopolsky M. Exercise testing as a diagnostic entity in mitochondrial myopathies. Mitochondrion. 2004;4(5-6):529-542. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
