Circadian regulation of developmental synaptogenesis via the hypocretinergic system
- PMID: 37268623
- PMCID: PMC10238477
- DOI: 10.1038/s41467-023-38973-w
Circadian regulation of developmental synaptogenesis via the hypocretinergic system
Abstract
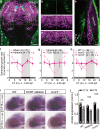
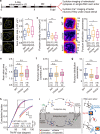
The circadian clock orchestrates a wide variety of physiological and behavioral processes, enabling animals to adapt to daily environmental changes, particularly the day-night cycle. However, the circadian clock's role in the developmental processes remains unclear. Here, we employ the in vivo long-term time-lapse imaging of retinotectal synapses in the optic tectum of larval zebrafish and reveal that synaptogenesis, a fundamental developmental process for neural circuit formation, exhibits circadian rhythm. This rhythmicity arises primarily from the synapse formation rather than elimination and requires the hypocretinergic neural system. Disruption of this synaptogenic rhythm, by impairing either the circadian clock or the hypocretinergic system, affects the arrangement of the retinotectal synapses on axon arbors and the refinement of the postsynaptic tectal neuron's receptive field. Thus, our findings demonstrate that the developmental synaptogenesis is under hypocretin-dependent circadian regulation, suggesting an important role of the circadian clock in neural development.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

