Pembrolizumab in brain metastases of diverse histologies: phase 2 trial results
- PMID: 37268724
- PMCID: PMC10644912
- DOI: 10.1038/s41591-023-02392-7
Pembrolizumab in brain metastases of diverse histologies: phase 2 trial results
Abstract
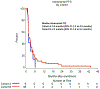
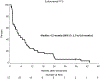
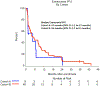
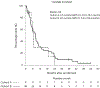
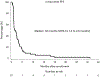
Brain metastases (BMs) are an emerging challenge in oncology due to increasing incidence and limited treatments. Here, we present results of a single-arm, open-label, phase 2 trial evaluating intracranial efficacy of pembrolizumab, a programmed cell death protein 1 inhibitor, in 9 patients with untreated BMs (cohort A) and 48 patients with recurrent and progressive BMs (cohort B) across different histologies. The primary endpoint was the proportion of patients achieving intracranial benefit, defined by complete response, partial response or stable disease. The primary endpoint was met with an intracranial benefit rate of 42.1% (90% confidence interval (CI): 31-54%). The median overall survival, a secondary endpoint, was 8.0 months (90% CI: 5.5-8.7 months) across both cohorts, 6.5 months (90% CI: 4.5-18.7 months) for cohort A and 8.1 months (90% CI: 5.3-9.6 months) for cohort B. Seven patients (12.3%), encompassing breast, melanoma and sarcoma histologies, had overall survival greater than 2 years. Thirty patients (52%; 90% CI: 41-64%) had one or more grade-3 or higher adverse events that were at least possibly treatment related. Two patients had grade-4 adverse events (cerebral edema) that were deemed at least possibly treatment related. These results suggest that programmed cell death protein 1 blockade may benefit a select group of patients with BMs, and support further studies to identify biomarkers and mechanisms of resistance. ClinicalTrials.gov identifier: NCT02886585.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures
References
-
- Gradishar WJ et al. Clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw 16, 310–320 (2018). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

