PARP Inhibitors in Ovarian Cancer: A Review
- PMID: 37268756
- PMCID: PMC10344972
- DOI: 10.1007/s11523-023-00970-w
PARP Inhibitors in Ovarian Cancer: A Review
Abstract
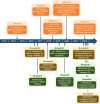
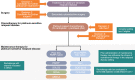
Poly(ADP-ribose) polymerase (PARP) inhibitors (PARPis) have transformed the ovarian cancer (OC) treatment landscape. This narrative review provides a comprehensive overview of data for the PARPis olaparib, niraparib, and rucaparib in patients with OC and discusses their role in disease management, with a focus on the use of PARPis as maintenance therapy in the United States (US). Olaparib was the first PARPi to be approved as first-line maintenance monotherapy in the US, with maintenance niraparib subsequently approved in the first-line setting. Data also support the efficacy of rucaparib as first-line maintenance monotherapy. PARPi maintenance combination therapy (olaparib plus bevacizumab) also provides benefit in patients with newly diagnosed advanced OC whose tumors tested positive for homologous recombination deficiency (HRD). Biomarker testing is critical in the newly diagnosed setting to identify patients most likely to benefit from PARPi maintenance therapy and guide treatment decisions. Clinical trial data support the use of PARPis (olaparib, niraparib, rucaparib) as second-line or later maintenance therapy in patients with platinum-sensitive relapsed OC. Although distinct differences in tolerability profile were observed between PARPis, they were generally well tolerated, with the majority of adverse events managed by dose modification. PARPis had no detrimental effect on patients' health-related quality of life. Real-world data support the use of PARPis in OC, although some differences between PARPis are apparent. Data from trials investigating novel combination strategies, such as PARPis plus immune checkpoint inhibitors, are awaited with interest; the optimal sequencing of novel therapies in OC remains to be established.
© 2023. The Author(s).
Conflict of interest statement
David M. O’Malley: Personal fees and/or advisory board/consulting fees from AbbVie, AdaptImmune, Agenus Inc., Arquer Diagnostics, Arcus Biosciences Inc., AstraZeneca, Atossa Therapeutics, Boston Biomedical, Cardiff Oncology, Celcuity, Clovis Oncology, Corcept Therapeutics, Duality Bio, Eisai, Elevar, Exelixis, Genentech Inc., Genelux, GlaxoSmithKline, GOG Foundation, Hoffmann-La Roche Inc., ImmunoGen Inc., Imvax, InterVenn, INXMED, IOVANCE Biotherapeutics, Janssen, Jazz Pharmaceuticals, Laekna, Leap Therapeutics, Inc., Luzsana Biotechology, Merck & Co, Merck Sharp & Dohme Corp., Mersana Therapeutics Inc., Myriad, Novartis, NovoCure, OncoC4 Inc., Onconova, Regeneron Pharmaceuticals Inc., RepImmune, R Pharm, Roche Diagnostics, Seattle Genetics (SeaGen), Sorrento, Sutro Biopharma, Tarveda Therapeutics, Toray, Trillium, Umoja, Verastem Inc., VBL Therapeutics, Vincerx Pharma, Xencor and Zentalis and research funding to his institution from AbbVie, Advaxis, Agenus Inc., Alkermes, Aravive Inc., Arcus Biosciences Inc., AstraZeneca, BeiGene USA Inc., Boston Biomedical, Bristol Myers Squibb, Clovis Oncology, Deciphera Pharma, Eisai, EMD Serono Inc., Exelixis, Genentech Inc., Genmab, GlaxoSmithKline, GOG Foundation, Hoffmann-La Roche Inc., ImmunoGen Inc., Incyte Corporation, IOVANCE Biotherapeutics, Karyopharm, Leap Therapeutics Inc., Ludwig Institute for Cancer Research, Merck & Co, Merck Sharp & Dohme Corp., Mersana Therapeutics Inc., NCI, Novartis, NovoCure, NRG Oncology, OncoC4 Inc., OncoQuest Inc., Pfizer Inc., Precision Therapeutics Inc., Prelude Therapeutics, Regeneron Pharmaceuticals Inc., RTOG, Rubius Therapeutics, Seattle Genetics (SeaGen), Sutro Biopharma, SWOG, TESARO, Verastem Inc. Thomas C. Krivak: Consulting fees, speaking fees and research support from AstraZeneca and GlaxoSmithKline; consulting fees and speaking fees from Myriad and Seagen/Genmab; and speaking fees and research support from Merck. Nashwa Kabil: Employment and stock ownership with AstraZeneca. Jiefen Munley: Employment and stock ownership with AstraZeneca. Kathleen N. Moore: Contracts from Genentech/Roche, Lilly Pharmaceuticals and PTC Therapeutics, for ovarian cancer investigator-initiated trials; consulting fees from IMab; payment to her institution for educational content in gynecological cancers from Onc Live, Physician Education Resource (PER), PRIME Oncology and Research to Practice; payment to her institution for advisory boards for use of assets in gynecological cancers from Alkemeres, Aravive, Blueprint Pharmaceuticals, Eisai, Genentech/Roche, Immunogen, Mersana, Mereo and VBL Therapeutics; participation on a data safety monitoring board for Incyte; payments to her institution for being an Associate Director of GOG partners and committee chair for NRG Ovarian Cancer.
Figures

References
-
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 1. 2022. Available at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed 2 Feb 2022.
-
- Genentech Inc. AVASTIN® (bevacizumab) injection, for intravenous use: prescribing information. 2022. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf. Accessed 5 Apr 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

