Non-adherence and non-persistence to intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy: a systematic review and meta-analysis
- PMID: 37269003
- PMCID: PMC10237080
- DOI: 10.1186/s13643-023-02261-x
Non-adherence and non-persistence to intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy: a systematic review and meta-analysis
Abstract
Background: Intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections play a key role in treating a range of macular diseases. The effectiveness of these therapies is dependent on patients' adherence (the extent to which a patient takes their medicines as per agreed recommendations from the healthcare provider) and persistence (continuation of the treatment for the prescribed duration) to their prescribed treatment regimens. The aim of this systematic review was to demonstrate the need for further investigation into the prevalence of, and factors contributing to, patient-led non-adherence and non-persistence, thus facilitating improved clinical outcomes.
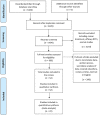
Methods: Systematic searches were conducted in Google Scholar, Web of Science, PubMed, MEDLINE, and the Cochrane Library. Studies in English conducted before February 2023 that reported the level of, and/or barriers to, non-adherence or non-persistence to intravitreal anti-VEGF ocular disease therapy were included. Duplicate papers, literature reviews, expert opinion articles, case studies, and case series were excluded following screening by two independent authors.
Results: Data from a total of 409,215 patients across 52 studies were analysed. Treatment regimens included pro re nata, monthly and treat-and-extend protocols; study durations ranged from 4 months to 8 years. Of the 52 studies, 22 included a breakdown of reasons for patient non-adherence/non-persistence. Patient-led non-adherence varied between 17.5 and 35.0% depending on the definition used. Overall pooled prevalence of patient-led treatment non-persistence was 30.0% (P = 0.000). Reasons for non-adherence/non-persistence included dissatisfaction with treatment results (29.9%), financial burden (19%), older age/comorbidities (15.5%), difficulty booking appointments (8.5%), travel distance/social isolation (7.9%), lack of time (5.8%), satisfaction with the perceived improvement in their condition (4.4%), fear of injection (4.0%), loss of motivation (4.0%), apathy towards eyesight (2.5%), dissatisfaction with facilities 2.3%, and discomfort/pain (0.3%). Three studies found non-adherence rates between 51.6 and 68.8% during the COVID-19 pandemic, in part due to fear of exposure to COVID-19 and difficulties travelling during lockdown.
Discussion: Results suggest high levels of patient-led non-adherence/non-persistence to anti-VEGF therapy, mostly due to dissatisfaction with treatment results, a combination of comorbidities, loss of motivation and the burden of travel. This study provides key information on prevalence and factors contributing to non-adherence/non-persistence in anti-VEGF treatment for macular diseases, aiding identification of at-risk individuals to improve real-world visual outcomes. Improvements in the literature can be achieved by establishing uniform definitions and standard timescales for what constitutes non-adherence/non-persistence.
Systematic review registration: PROSPERO CRD42020216205.
Keywords: Anti-VEGF; COVID-19; Intravitreal; Macular; Meta-analysis; Non-adherence; Non-persistence.
© 2023. Crown.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–1411. doi: 10.1016/j.ophtha.2012.04.015. - DOI - PubMed
-
- Royal National Institute of Blind People. Anti-VEGF treatment [Internet]. RNIB; 2022. [updated 2022 Sep 1; cited 2022 Sep 19]. Available from: https://www.rnib.org.uk/your-eyes/eye-conditions-az/anti-vegf-treatment/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

