The improving Medication Adherence in Adolescents and young adults following Liver Transplantation (iMALT) multisite trial: Design and trial implementation considerations
- PMID: 37269062
- PMCID: PMC10524899
- DOI: 10.1177/17407745231176834
The improving Medication Adherence in Adolescents and young adults following Liver Transplantation (iMALT) multisite trial: Design and trial implementation considerations
Abstract
Background/aims: Medication non-adherence is a leading cause of transplant rejection, organ loss, and death; yet no rigorous controlled study to date has shown compelling clinical benefits from an adherence-improving intervention. Non-adherent patients are less likely to participate in trials, and therefore, most studies enroll a majority of adherent patients who do not stand to benefit from the intervention, as they do not have the condition (non-adherence) under investigation. The improving Medication Adherence in adolescent Liver Transplant recipients trial specifically targets non-adherent patients to investigate whether a remote intervention to improve adherence results in reduced incidence of biopsy-confirmed rejection.
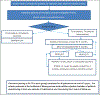
Methods: Improving Medication Adherence in adolescent Liver Transplant is a randomized single-blind controlled multisite, multinational National Institutes of Health-funded trial involving 13 pediatric transplant centers in the United States and Canada. An innovative, objective adherence biomarker-the Medication Level Variability Index, which is the standard deviation of a series of medication blood levels for each patient, is used to identify non-adherent patients at risk for rejection. The index is computed using electronic health record information for all potentially eligible patients based on repeated reviews of the entire clinic's roster. Identified patients, after consent, are randomized to intervention versus control (treatment as usual) arms. The remote intervention is delivered for 2 years by trained interventionists who reside in various locations in the United States. The primary outcome is the incidence of biopsy-confirmed acute cellular rejection, as confirmed by a majority vote of three pathologists who are masked to the study allocation and clinical information.
Discussion: Improving Medication Adherence in adolescent Liver Transplant includes several innovative design elements. The use of a validated, objective adherence index to survey a large cohort of transplant recipients allows the teams to avoid bias inherent in both convenience sampling and referral-based recruitment and enroll only patients whose computed index indicates substantially increased risk of rejection. The remote intervention paradigm helps to engage patients who are by definition hard to engage. The use of an objective, masked medical (rather than behavioral) outcome measure reduces the likelihood of biases related to clinical information and ensures broad acceptance by the field. Finally, monitoring for potential adverse events related to increased medication exposure due to the adherence intervention acknowledges that a successful intervention (increasing adherence) could have detrimental side effects via increased exposure to and potential toxicity of the medication. Such monitoring is almost never attempted in clinical trials evaluating adherence interventions.
Keywords: Pediatric transplant; biomarker; randomized clinical trial; telemetric intervention.
Conflict of interest statement
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
-
- Fredericks EM, Lopez MJ, Magee JC, et al. Psychological functioning, nonadherence and health outcomes after pediatric liver transplantation. Am J Transplant 2007; 7(8): 1974–1983. - PubMed
-
- Shemesh E, Shneider BL, Savitzky JK, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics 2004; 113: 825–832. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical