Management of pharmacovigilance during the COVID-19 pandemic crisis by the safety department of an academic sponsor: Lessons learnt and challenges from the EU DisCoVeRy clinical trial
- PMID: 37269068
- PMCID: PMC10238756
- DOI: 10.1002/prp2.1072
Management of pharmacovigilance during the COVID-19 pandemic crisis by the safety department of an academic sponsor: Lessons learnt and challenges from the EU DisCoVeRy clinical trial
Abstract
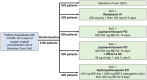
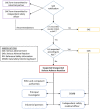
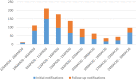
The current COVID-19 pandemic was an exceptional health situation, including for drug use. As there was no known effective drug for COVID-19 at the beginning of the pandemic, different drug candidates were proposed. In this article, we present the challenges for an academic Safety Department to manage the global safety of a European trial during the pandemic. The National Institute for Health and Medical Research (Inserm) conducted a European multicenter, open-label, randomized, controlled trial involving three repurposed and one-in development drugs (lopinavir/ritonavir, IFN-β1a, hydroxychloroquine, and remdesivir) in adults hospitalized with COVID-19. From 25 March 2020 to 29 May 2020, the Inserm Safety Department had to manage 585 Serious Adverse Events (SAEs) initial notification and 396 follow-up reports. The Inserm Safety Department's staff was mobilized to manage these SAEs and to report Expedited safety reports to the competent authorities within the legal timeframes. More than 500 queries were sent to the investigators due to a lack of or incoherent information on SAE forms. At the same time, the investigators were overwhelmed by the management of patients suffering from COVID-19 infection. These particular conditions of missing data and lack of accurate description of adverse events made evaluation of the SAEs very difficult, particularly the assessment of the causal role of each investigational medicinal product. In parallel, working difficulties were accentuated by the national lockdown, frequent IT tool dysfunctions, delayed implementation of monitoring and the absence of automatic alerts for SAE form modification. Although COVID-19 is a confounding factor per se, the delay in and quality of SAE form completion and the real-time medical analysis by the Inserm Safety Department were major issues in the quick identification of potential safety signals. To conduct a high-quality clinical trial and ensure patient safety, all stakeholders must take their roles and responsibilities.
Trial registration: ClinicalTrials.gov NCT 04315948.
Keywords: COVID-19; EU-RESPONSE; adverse event; pandemic crisis; pharmacovigilance.
© 2023 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
No author declared a conflict of interest in relation to the submitted work. C. Delmas reports receipt of drugs from Gilead Sciences, Inc., Sanofi, Merck Group, AbbVie for Inserm, outside the submitted work. J. Saillard reports receipt of drugs from Gilead Sciences, Inc., Sanofi, Merck Group, AbbVie for Inserm, outside the submitted work. M. Dumousseaux reports receipt of drugs from Gilead Sciences, Inc., Sanofi, Merck Group, AbbVie for Inserm, outside the submitted work. S. Le Mestre reports receipt of drugs from Gilead Sciences, Inc., Sanofi, Merck Group, AbbVie for Inserm, outside the submitted work. A. Ferrane reports receipt of drugs from Gilead Sciences, Inc., Sanofi, Merck Group, AbbVie for Inserm, outside the submitted work. C. Burdet reports consulting fees from Mylan, Da Volterra, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for 4Living Biotech, outside the submitted work. F. Ader reports grants from The French Health Ministry, European Community, outside the submitted work. M. Hites reports grants from the Belgian Centre for Knowledge (KCE), the Fonds Erasme‐COVID‐Université Libre de Bruxelles, outside the submitted work; grants from the EU‐Horizon program, outside the submitted work; support for ECCMID congress 2021 from Pfizer, outside the submitted work; working as co‐leader of the Belgian Guidelines on Therapeutics for COVID‐19, outside the submitted work; acting as a president for the Belgian Society of Clinical Microbiology and Infectious Diseases, outside the submitted work. J. Poissy reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Gilead, outside the submitted work; patents planned, issued or pending from Gilead, outside the submitted work. C. Andrejak reports grants from the FEDER, outside the submitted work; support for ERS Conference from Homeperf, outside the submitted work; acting as member of Covid group for the French High council of Public Health and acting as responsible of the Scientific council for the French Society of Respiratory Diseases, outside the submitted work. J.A. Paiva reports consulting fees from MSD, Jansen, Pfizer, Astra‐Zeneca, outside the submitted work; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Gilead, Cepheid, AOP Orphan Pharmaceuticals, outside the submitted work. R. Greil reports consulting fees from Celgene, Novartis, Roche, BMS, Takeda, Abbvie, MSD, Janssen, Astra‐Zeneca, Merck, Gilead, Daiichi Sankyo, Sanofi, outside the submitted work; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Celgene, Roche, BMS, Takeda, Abbvie, Sandoz, MSD, Novartis, Astra‐Zeneca, Merck, Gilead, Daiichi Sankyo, Sanofi, Amgen, outside the submitted work; support for attending meeting and/or travel from Celgene, Roche, BMS, Abbvie, MSD, Novartis, Astra‐Zeneca, Gilead, Daiichi Sankyo, Amgen, Janssen, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Celgene, Novartis, Roche, BMS, Takeda, Abbvie, Astra‐Zeneca, MSD, Janssen, Merck, Gilead, Daiichi Sankyo, Sanofi, outside the submitted work; research funding from Celgene, Roche, Merck, Takeda, Astra‐Zeneca, Novartis, Amgen, BMS, MSD, Sandoz, Abbvie, Gilead, Daiichi Sankyo, outside the submitted work. D. Costagliola reports grants or contracts from any entity from Janssen for Inserm, outside the submitted work; lectures fees from Janssen and Gilead, outside the submitted work; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Gilead, outside the submitted work. All other authors declare no competing interests.
Figures
References
-
- World Health Organization . Coronavirus disease (COVID‐19). Technical guidance. 2020. https://www.who.int/fr/emergencies/diseases/novel‐coronavirus‐2019/techn..., 30 Nov 2020
-
- de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA‐approved compound library identifies four small‐molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875‐4884. doi: 10.1128/AAC.03011-14 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials