rTMS ameliorates depressive-like behaviors and regulates the gut microbiome and medium- and long-chain fatty acids in mice exposed to chronic unpredictable mild stress
- PMID: 37269082
- PMCID: PMC10580350
- DOI: 10.1111/cns.14287
rTMS ameliorates depressive-like behaviors and regulates the gut microbiome and medium- and long-chain fatty acids in mice exposed to chronic unpredictable mild stress
Abstract
Introduction: Repetitive transcranial magnetic stimulation (rTMS) is a clinically useful therapy for depression. However, the effects of rTMS on the metabolism of fatty acids (FAs) and the composition of gut microbiota in depression are not well established.
Methods: Mice received rTMS (15 Hz, 1.26 T) for seven consecutive days after exposure to chronic unpredictable mild stress (CUMS). The subsequent depressive-like behaviors, the composition of gut microbiota of stool samples, as well as medium- and long-chain fatty acids (MLCFAs) in the plasma, prefrontal cortex (PFC), and hippocampus (HPC) were evaluated.
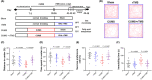
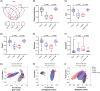
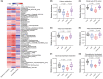
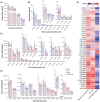
Results: CUMS induced remarkable changes in gut microbiotas and fatty acids, specifically in community diversity of gut microbiotas and PUFAs in the brain. 15 Hz rTMS treatment alleviates depressive-like behaviors and partially normalized CUMS induced alterations of microbiotas and MLCFAs, especially the abundance of Cyanobacteria, Actinobacteriota, and levels of polyunsaturated fatty acids (PUFAs) in the hippocampus and PFC.
Conclusion: These findings revealed that the modulation of gut microbiotas and PUFAs metabolism might partly contribute to the antidepressant effect of rTMS.
Keywords: CUMS; gut microbiota; medium- and long-chain fatty acids; rTMS.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299‐2312. - PubMed
-
- Locher C, Koechlin H, Zion SR, et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin‐norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta‐analysis. JAMA Psychiat. 2017;74(10):1011‐1020. - PMC - PubMed
-
- Pu Y, Tan Y, Qu Y, et al. A role of the subdiaphragmatic vagus nerve in depression‐like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock‐out mice with depression‐like phenotypes. Brain Behav Immun. 2021;94:318‐326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

