Transcutaneous spinal stimulation in people with and without spinal cord injury: Effect of electrode placement and trains of stimulation on threshold intensity
- PMID: 37269156
- PMCID: PMC10238786
- DOI: 10.14814/phy2.15692
Transcutaneous spinal stimulation in people with and without spinal cord injury: Effect of electrode placement and trains of stimulation on threshold intensity
Abstract
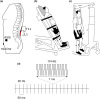
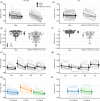
Transcutaneous spinal cord stimulation (TSS) is purported to improve motor function in people after spinal cord injury (SCI). However, several methodology aspects are yet to be explored. We investigated whether stimulation configuration affected the intensity needed to elicit spinally evoked motor responses (sEMR) in four lower limb muscles bilaterally. Also, since stimulation intensity for therapeutic TSS (i.e., trains of stimulation, typically delivered at 15-50 Hz) is sometimes based on the single-pulse threshold intensity, we compared these two stimulation types. In non-SCI participants (n = 9) and participants with a SCI (n = 9), three different electrode configurations (cathode-anode); L1-midline (below the umbilicus), T11-midline and L1-ASIS (anterior superior iliac spine; non-SCI only) were compared for the sEMR threshold intensity using single pulses or trains of stimulation which were recorded in the vastus medialis, medial hamstring, tibialis anterior, medial gastrocnemius muscles. In non-SCI participants, the L1-midline configuration showed lower sEMR thresholds compared to T11-midline (p = 0.002) and L1-ASIS (p < 0.001). There was no difference between T11-midline and L1-midline for participants with SCI (p = 0.245). Spinally evoked motor response thresholds were ~13% lower during trains of stimulation compared to single pulses in non-SCI participants (p < 0.001), but not in participants with SCI (p = 0.101). With trains of stimulation, threshold intensities were slightly lower and the incidence of sEMR was considerably lower. Overall, stimulation threshold intensities were generally lower with the L1-midline electrode configuration and is therefore preferred. While single-pulse threshold intensities may overestimate threshold intensities for therapeutic TSS, tolerance to trains of stimulation will be the limiting factor in most cases.
Keywords: spinal cord injury; transcutaneous spinal stimulation.
© 2023 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Figures




Similar articles
-
Differential effects of stimulation waveform and intensity on the neural structures activated by lumbar transcutaneous spinal cord stimulation.J Neurophysiol. 2025 Feb 1;133(2):447-463. doi: 10.1152/jn.00266.2024. Epub 2024 Dec 24. J Neurophysiol. 2025. PMID: 39718492
-
Bipolar transcutaneous spinal stimulation evokes short-latency reflex responses in human lower limbs alike standard unipolar electrode configuration.J Neurophysiol. 2020 Oct 1;124(4):1072-1082. doi: 10.1152/jn.00433.2020. Epub 2020 Aug 26. J Neurophysiol. 2020. PMID: 32845202
-
Optimizing transcutaneous spinal stimulation: excitability of evoked spinal reflexes is dependent on electrode montage.J Neuroeng Rehabil. 2025 Jan 6;22(1):2. doi: 10.1186/s12984-024-01524-5. J Neuroeng Rehabil. 2025. PMID: 39762915 Free PMC article. Clinical Trial.
-
Transcutaneous Spinal Cord Stimulation and Motor Rehabilitation in Spinal Cord Injury: A Systematic Review.Neurorehabil Neural Repair. 2020 Jan;34(1):3-12. doi: 10.1177/1545968319893298. Epub 2019 Dec 20. Neurorehabil Neural Repair. 2020. PMID: 31858871
-
Transcutaneous spinal cord stimulation and motor responses in individuals with spinal cord injury: A methodological review.PLoS One. 2021 Nov 18;16(11):e0260166. doi: 10.1371/journal.pone.0260166. eCollection 2021. PLoS One. 2021. PMID: 34793572 Free PMC article.
Cited by
-
Noninvasive Electrical Modalities to Alleviate Respiratory Deficits Following Spinal Cord Injury.Life (Basel). 2024 Dec 13;14(12):1657. doi: 10.3390/life14121657. Life (Basel). 2024. PMID: 39768364 Free PMC article. Review.
-
Optimizing Transcutaneous Spinal Cord Stimulation: An Exploratory Study on the Role of Electrode Montages and Stimulation Intensity on Reflex Pathway Modulation.Bioengineering (Basel). 2025 Apr 12;12(4):410. doi: 10.3390/bioengineering12040410. Bioengineering (Basel). 2025. PMID: 40281770 Free PMC article.
References
-
- Al'joboori, Y. , Massey, S. J. , Knight, S. L. , Donaldson, N. N. , & Duffell, L. D. (2020). The effects of adding transcutaneous spinal cord stimulation (TSCS) to sit‐to‐stand training in people with spinal cord injury: A pilot study. Journal of Clinical Medicine, 9, 2765. 10.3390/jcm9092765 - DOI - PMC - PubMed
-
- Bedi, P. K. , & Arumugam, N. (2016). Tapping the neural circuitry: Surface spinal stimulation in spinal cord injury: A case report. JESP, 12, 69–75. 10.18376//2016/v12i1/86815 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

