Age- and sex-dependent alterations in primary somatosensory cortex neuronal calcium network dynamics during locomotion
- PMID: 37269157
- PMCID: PMC10410056
- DOI: 10.1111/acel.13898
Age- and sex-dependent alterations in primary somatosensory cortex neuronal calcium network dynamics during locomotion
Abstract
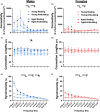
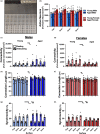
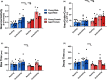
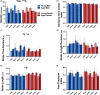
Over the past 30 years, the calcium (Ca2+ ) hypothesis of brain aging has provided clear evidence that hippocampal neuronal Ca2+ dysregulation is a key biomarker of aging. Age-dependent Ca2+ -mediated changes in intrinsic excitability, synaptic plasticity, and activity have helped identify some of the mechanisms engaged in memory and cognitive decline based on work done mostly at the single-cell level and in the slice preparation. Recently, our lab identified age- and Ca2+ -related neuronal network dysregulation in the cortex of the anesthetized animal. Still, investigations in the awake animal are needed to test the generalizability of the Ca2+ hypothesis of brain aging. Here, we used in vigilo two-photon imaging in ambulating mice, to image GCaMP8f in the primary somatosensory cortex (S1), during ambulation and at rest. We investigated aging- and sex-related changes in neuronal networks in the C56BL/6J mouse. Following imaging, gait behavior was characterized to test for changes in locomotor stability. During ambulation, in both young adult and aged mice, an increase in network connectivity and synchronicity was noted. An age-dependent increase in synchronicity was seen in ambulating aged males only. Additionally, females displayed increases in the number of active neurons, Ca2+ transients, and neuronal activity compared to males, particularly during ambulation. These results suggest S1 Ca2+ dynamics and network synchronicity are likely contributors of locomotor stability. We believe this work raises awareness of age- and sex-dependent alterations in S1 neuronal networks, perhaps underlying the increase in falls with age.
Keywords: Ca2+; behavior; locomotor stability; mice; neuronal network; neuroscience; somatosensation; two-photon imaging.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Sensitivity of the S1 neuronal calcium network to insulin and Bay-K 8644 in vivo: Relationship to gait, motivation, and aging processes.Aging Cell. 2022 Jul;21(7):e13661. doi: 10.1111/acel.13661. Epub 2022 Jun 19. Aging Cell. 2022. PMID: 35717599 Free PMC article.
-
Aberrant cortical activity, functional connectivity, and neural assembly architecture after photothrombotic stroke in mice.Elife. 2024 Apr 30;12:RP90080. doi: 10.7554/eLife.90080. Elife. 2024. PMID: 38687189 Free PMC article.
-
Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity.J Neurosci. 2001 Dec 15;21(24):9744-56. doi: 10.1523/JNEUROSCI.21-24-09744.2001. J Neurosci. 2001. PMID: 11739583 Free PMC article.
-
Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity.Hippocampus. 1997;7(6):602-12. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. Hippocampus. 1997. PMID: 9443057 Review.
-
Ageing, hippocampal synaptic activity and magnesium.Magnes Res. 2006 Sep;19(3):199-215. Magnes Res. 2006. PMID: 17172010 Review.
Cited by
-
Astrovascular decoupling in awake 5×FAD mice is associated with reduced astrocytic calcium.Alzheimers Dement. 2025 Aug;21(8):e70564. doi: 10.1002/alz.70564. Alzheimers Dement. 2025. PMID: 40799120 Free PMC article.
-
The Impact of Overexpression of the Mouse Ortholog of CACNA1C on Behavior and Cortical Dynamics.Biol Psychiatry Glob Open Sci. 2025 May 22;5(5):100537. doi: 10.1016/j.bpsgos.2025.100537. eCollection 2025 Sep. Biol Psychiatry Glob Open Sci. 2025. PMID: 40678692 Free PMC article.
References
-
- Beauchet, O. , Annweiler, C. , Callisaya, M. L. , De Cock, A. M. , Helbostad, J. L. , Kressig, R. W. , Srikanth, V. , Steinmetz, J. P. , Blumen, H. M. , Verghese, J. , & Allali, G. (2016). Poor gait performance and prediction of dementia: Results from a meta‐analysis. Journal of the American Medical Directors Association, 17(6), 482–490. 10.1016/j.jamda.2015.12.092 - DOI - PMC - PubMed
-
- Beauchet, O. , Launay, C. P. , Sekhon, H. , Montembeault, M. , & Allali, G. (2019). Association of hippocampal volume with gait variability in pre‐dementia and dementia stages of Alzheimer disease: Results from a cross‐sectional study. Experimental Gerontology, 115, 55–61. 10.1016/j.exger.2018.11.010 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

