The expression of ceruloplasmin in astrocytes is essential for postnatal myelination and myelin maintenance in the adult brain
- PMID: 37269227
- PMCID: PMC10599212
- DOI: 10.1002/glia.24424
The expression of ceruloplasmin in astrocytes is essential for postnatal myelination and myelin maintenance in the adult brain
Abstract
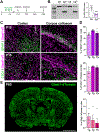
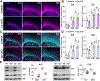
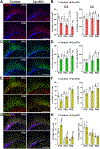
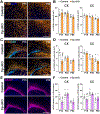
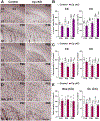
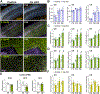
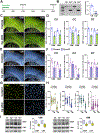
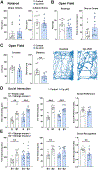
Ceruloplasmin (Cp) is a ferroxidase enzyme that is essential for cell iron efflux. The absence of this protein in humans and rodents produces progressive neurodegeneration with brain iron accumulation. Astrocytes express high levels of Cp and iron efflux from these cells has been shown to be central for oligodendrocyte maturation and myelination. To explore the role of astrocytic Cp in brain development and aging we generated a specific conditional KO mouse for Cp in astrocytes (Cp cKO). Deletion of Cp in astrocytes during the first postnatal week induced hypomyelination and a significant delay in oligodendrocyte maturation. This abnormal myelin synthesis was exacerbated throughout the first two postnatal months and accompanied by a reduction in oligodendrocyte iron content, as well as an increase in brain oxidative stress. In contrast to young animals, deletion of astrocytic Cp at 8 months of age engendered iron accumulation in several brain areas and neurodegeneration in cortical regions. Aged Cp cKO mice also showed myelin loss and oxidative stress in oligodendrocytes and neurons, and at 18 months of age, developed abnormal behavioral profiles, including deficits in locomotion and short-term memory. In summary, our results demonstrate that iron efflux-mediated by astrocytic Cp-is essential for both early oligodendrocyte maturation and myelin integrity in the mature brain. Additionally, our data suggest that astrocytic Cp activity is central to prevent iron accumulation and iron-induced oxidative stress in the aging CNS.
Keywords: astrocytes; ceruloplasmin; iron; myelination; oligodendrocytes.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
Figures



Similar articles
-
Ceruloplasmin deletion in myelinating glial cells induces myelin disruption and oxidative stress in the central and peripheral nervous systems.Redox Biol. 2021 Oct;46:102118. doi: 10.1016/j.redox.2021.102118. Epub 2021 Aug 27. Redox Biol. 2021. PMID: 34474395 Free PMC article.
-
Multi-copper ferroxidase deficiency leads to iron accumulation and oxidative damage in astrocytes and oligodendrocytes.Sci Rep. 2019 Jul 1;9(1):9437. doi: 10.1038/s41598-019-46019-9. Sci Rep. 2019. PMID: 31263155 Free PMC article.
-
H-ferritin expression in astrocytes is necessary for proper oligodendrocyte development and myelination.Glia. 2021 Dec;69(12):2981-2998. doi: 10.1002/glia.24083. Epub 2021 Aug 30. Glia. 2021. PMID: 34460113 Free PMC article.
-
Iron Metabolism in Oligodendrocytes and Astrocytes, Implications for Myelination and Remyelination.ASN Neuro. 2020 Jan-Dec;12:1759091420962681. doi: 10.1177/1759091420962681. ASN Neuro. 2020. PMID: 32993319 Free PMC article. Review.
-
Ceruloplasmin in neurodegenerative diseases.Biochem Soc Trans. 2008 Dec;36(Pt 6):1277-81. doi: 10.1042/BST0361277. Biochem Soc Trans. 2008. PMID: 19021540 Review.
Cited by
-
Ceruloplasmin and Ferritin Changes in Ocular Fluids from Patients with Vitreoretinal Diseases: Relation with Neuroinflammation and Drusen Formation.Int J Mol Sci. 2025 Jun 30;26(13):6307. doi: 10.3390/ijms26136307. Int J Mol Sci. 2025. PMID: 40650085 Free PMC article.
-
Role of iron in brain development, aging, and neurodegenerative diseases.Ann Med. 2025 Dec;57(1):2472871. doi: 10.1080/07853890.2025.2472871. Epub 2025 Mar 4. Ann Med. 2025. PMID: 40038870 Free PMC article. Review.
-
Inhibitory maturation and ocular dominance plasticity in mouse visual cortex require astrocyte CB1 receptors.iScience. 2024 Nov 17;27(12):111410. doi: 10.1016/j.isci.2024.111410. eCollection 2024 Dec 20. iScience. 2024. PMID: 39687028 Free PMC article.
-
Ferritin loss in astrocytes reduces spinal cord oxidative stress and demyelination in the experimental autoimmune encephalomyelitis (EAE) model.Glia. 2024 Dec;72(12):2327-2343. doi: 10.1002/glia.24616. Epub 2024 Sep 3. Glia. 2024. PMID: 39228110
-
Mammalian copper homeostasis: physiological roles and molecular mechanisms.Physiol Rev. 2025 Jan 1;105(1):441-491. doi: 10.1152/physrev.00011.2024. Epub 2024 Aug 22. Physiol Rev. 2025. PMID: 39172219 Free PMC article. Review.
References
-
- Abbott NJ, Rönnbäck L, Hansson E (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7: 41–53. - PubMed
-
- Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A (2003). An alphasyntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci USA 100: 2106–2111. - PMC - PubMed
-
- Ayton S, Lei P, Bush AI (2013a). Metallostasis in Alzheimer’s disease. Free Radic Biol Med 62: 76–89. - PubMed
-
- Ayton S, Lei P, Duce JA, Wong BX, Sedjahtera A, Adlard PA, Bush AI, Finkelstein DI (2013b). Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann Neurol 73: 554–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

