The Microbiome in Advanced Melanoma: Where Are We Now?
- PMID: 37269504
- PMCID: PMC11090495
- DOI: 10.1007/s11912-023-01431-3
The Microbiome in Advanced Melanoma: Where Are We Now?
Abstract
Purpose of review: This review summarizes recent data linking gut microbiota composition to ICI outcomes and gut microbiota-specific interventional clinical trials in melanoma.
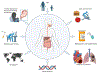
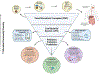
Recent findings: Preclinical and clinical studies have demonstrated the effects of the gut microbiome modulation upon ICI response in advanced melanoma, with growing evidence supporting the ability of the gut microbiome to restore or improve ICI response in advanced melanoma through dietary fiber, probiotics, and FMT. Immune checkpoint inhibitors (ICI) targeting the PD-1, CTLA-4, and LAG-3 negative regulatory checkpoints have transformed the management of melanoma. ICIs are FDA-approved in advanced metastatic disease, stage III resected melanoma, and high-risk stage II melanoma and are being investigated more recently in the management of high-risk resectable melanoma in the peri-operative setting. The gut microbiome has emerged as an important tumor-extrinsic modulator of both response and immune-related adverse event (irAE) development in ICI-treated cancer in general, and melanoma in particular.
Keywords: Cytotoxic T-lymphocyte antigen-4 (CTLA-4); Gut microbiome; Immunotherapy; Melanoma; Programmed death-1 (PD-1).
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflict of Interest Statement:
D.D.F and D.H. reports no conflicts of interest
D.D. reports the following disclosures:
Grants/Research Support (institutional): Arcus, CellSight Technologies, Immunocore, Merck, Regeneron Pharmaceuticals Inc., Tesaro/GSK
Consultant: ACM Bio, Ascendis Pharma, Clinical Care Options (CCO), Gerson Lehrman Group (GLG), Merck, Medical Learning Group (MLG), Xilio Therapeutics
CE Speakers’ Bureau: Castle Biosciences.
Stockholder: None.
Intellectual Property:
US Patent 63/124,231, “Compositions and Methods for Treating Cancer”, Dec 11, 2020
US Patent 63/208,719, “Compositions and Methods For Determining Responsiveness to Immune Checkpoint Inhibitors (ICI), Increasing Effectiveness of ICI and Treating Cancer”, June 9, 2021
Figures
Similar articles
-
Conversion of unresponsiveness to immune checkpoint inhibition by fecal microbiota transplantation in patients with metastatic melanoma: study protocol for a randomized phase Ib/IIa trial.BMC Cancer. 2022 Dec 30;22(1):1366. doi: 10.1186/s12885-022-10457-y. BMC Cancer. 2022. PMID: 36585700 Free PMC article.
-
Gut microbiome features associate with immune checkpoint inhibitor response in individuals with non-melanoma skin cancers: an exploratory study.Microbiol Spectr. 2025 Mar 4;13(3):e0255924. doi: 10.1128/spectrum.02559-24. Epub 2025 Feb 3. Microbiol Spectr. 2025. PMID: 39898646 Free PMC article.
-
Facts and Hopes for Gut Microbiota Interventions in Cancer Immunotherapy.Clin Cancer Res. 2022 Oct 14;28(20):4370-4384. doi: 10.1158/1078-0432.CCR-21-1129. Clin Cancer Res. 2022. PMID: 35748749 Free PMC article. Review.
-
The Gastrointestinal Microbiome and Immune Checkpoint Inhibitors: A Review of Human Interventional Studies Among Melanoma Patients.J Drugs Dermatol. 2024 Feb 1;23(2):78-84. doi: 10.36849/JDD.7674. J Drugs Dermatol. 2024. PMID: 38306142 Review.
-
TIGIT+ NK cells in combination with specific gut microbiota features predict response to checkpoint inhibitor therapy in melanoma patients.BMC Cancer. 2023 Nov 28;23(1):1160. doi: 10.1186/s12885-023-11551-5. BMC Cancer. 2023. PMID: 38017389 Free PMC article.
Cited by
-
Microbiomes, Their Function, and Cancer: How Metatranscriptomics Can Close the Knowledge Gap.Int J Mol Sci. 2023 Sep 7;24(18):13786. doi: 10.3390/ijms241813786. Int J Mol Sci. 2023. PMID: 37762088 Free PMC article. Review.
-
Therapeutic potential of fecal microbiota transplantation in colorectal cancer based on gut microbiota regulation: from pathogenesis to efficacy.Therap Adv Gastroenterol. 2025 Mar 18;18:17562848251327167. doi: 10.1177/17562848251327167. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40104324 Free PMC article. Review.
References
-
- Guy GP, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and Costs of Skin Cancer Treatment in the U.S., 2002−2006 and 2007−2011. American Journal of Preventive Medicine [Internet]. 2015. [cited 2022 Jun 9];48:183–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0749379714005108 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials