Mechanical loading of the ventricular wall as a spatial indicator for periventricular white matter degeneration
- PMID: 37269602
- PMCID: PMC10266836
- DOI: 10.1016/j.jmbbm.2023.105921
Mechanical loading of the ventricular wall as a spatial indicator for periventricular white matter degeneration
Abstract
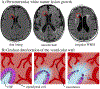
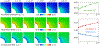
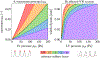
Progressive white matter degeneration in periventricular and deep white matter regions appears as white matter hyperintensities (WMH) on MRI scans. To date, periventricular WMHs are often associated with vascular dysfunction. Here, we demonstrate that ventricular inflation resulting from cerebral atrophy and hemodynamic pulsation with every heartbeat leads to a mechanical loading state of periventricular tissues that significantly affects the ventricular wall. Specifically, we present a physics-based modeling approach that provides a rationale for ependymal cell involvement in periventricular WMH formation. Building on eight previously created 2D finite element brain models, we introduce novel mechanomarkers for ependymal cell loading and geometric measures that characterize lateral ventricular shape. We show that our novel mechanomarkers, such as maximum ependymal cell deformations and maximum curvature of the ventricular wall, spatially overlap with periventricular WMH locations and are sensitive predictors for WMH formation. We also explore the role of the septum pellucidum in mitigating mechanical loading of the ventricular wall by constraining the radial expansion of the lateral ventricles during loading. Our models consistently show that ependymal cells are stretched thin only in the horns of the ventricles irrespective of ventricular shape. We therefore pose that periventricular WMH etiology is strongly linked to the deterioration of the over-stretched ventricular wall resulting in CSF leakage into periventricular white matter. Subsequent secondary damage mechanisms, including vascular degeneration, exacerbate lesion formation and lead to progressive growth into deep white matter regions.
Keywords: Ependymal cells; Finite element simulations; Lateral ventricles; Tissue damage; White matter hyperintensities.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Johannes Weickenmeier reports financial support was provided by National Institutes of Health. Henry Rusinek reports financial support was provided by National Institutes of Health.
Figures




Similar articles
-
A multiphysics model to predict periventricular white matter hyperintensity growth during healthy brain aging.Brain Multiphys. 2023 Dec;5:100072. doi: 10.1016/j.brain.2023.100072. Epub 2023 May 26. Brain Multiphys. 2023. PMID: 37546181 Free PMC article.
-
3D finite-element brain modeling of lateral ventricular wall loading to rationalize periventricular white matter hyperintensity locations.Eng Comput. 2022 Oct;38(5):3939-3955. doi: 10.1007/s00366-022-01700-y. Epub 2022 Jul 19. Eng Comput. 2022. PMID: 37485473 Free PMC article.
-
Peak ependymal cell stretch overlaps with the onset locations of periventricular white matter lesions.Sci Rep. 2021 Nov 9;11(1):21956. doi: 10.1038/s41598-021-00610-1. Sci Rep. 2021. PMID: 34753951 Free PMC article.
-
Can white matter hyperintensities based Fazekas visual assessment scales inform about Alzheimer's disease pathology in the population?Alzheimers Res Ther. 2024 Jul 10;16(1):157. doi: 10.1186/s13195-024-01525-5. Alzheimers Res Ther. 2024. PMID: 38987827 Free PMC article.
-
Three-tissue compositional analysis reveals in-vivo microstructural heterogeneity of white matter hyperintensities following stroke.Neuroimage. 2020 Sep;218:116869. doi: 10.1016/j.neuroimage.2020.116869. Epub 2020 Apr 22. Neuroimage. 2020. PMID: 32334092
Cited by
-
A multiphysics model to predict periventricular white matter hyperintensity growth during healthy brain aging.Brain Multiphys. 2023 Dec;5:100072. doi: 10.1016/j.brain.2023.100072. Epub 2023 May 26. Brain Multiphys. 2023. PMID: 37546181 Free PMC article.
-
Multiciliated ependymal cells: an update on biology and pathology in the adult brain.Acta Neuropathol. 2024 Sep 10;148(1):39. doi: 10.1007/s00401-024-02784-0. Acta Neuropathol. 2024. PMID: 39254862 Review.
-
Segmenting mechanically heterogeneous domains via unsupervised learning.Biomech Model Mechanobiol. 2024 Feb;23(1):349-372. doi: 10.1007/s10237-023-01779-2. Epub 2024 Jan 13. Biomech Model Mechanobiol. 2024. PMID: 38217746
References
-
- Fazekas F, Kleinert R, Offenbacher H, Pathologic correlates of incidental mri white matter signal hyperintensities, Neurology 43 (1993) 1683–1683. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

