Direct comparison of clinical diagnostic sensitivity of saliva from buccal swabs versus combined oro-/nasopharyngeal swabs in the detection of SARS-CoV-2 B.1.1.529 Omicron
- PMID: 37269606
- PMCID: PMC10207859
- DOI: 10.1016/j.jcv.2023.105496
Direct comparison of clinical diagnostic sensitivity of saliva from buccal swabs versus combined oro-/nasopharyngeal swabs in the detection of SARS-CoV-2 B.1.1.529 Omicron
Abstract
Background/purpose: While current guidelines recommend the use of respiratory tract specimens for the direct detection of SARS-CoV-2 infection, saliva has recently been suggested as preferred sample type for the sensitive detection of SARS-CoV-2 B.1.1.529 (Omicron). By comparing saliva collected using buccal swabs and oro-/nasopharyngeal swabs from patients hospitalized due to COVID-19, we aimed at identifying potential differences in virus detection sensitivity between these sample types.
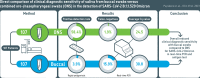
Methods: We compare the clinical diagnostic sensitivity of paired buccal swabs and combined oro-/nasopharyngeal swabs from hospitalized, symptomatic COVID-19 patients collected at median six days after symptom onset by real-time polymerase chain reaction (PCR) and antigen test.
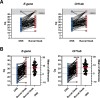
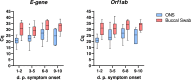
Results: Of the tested SARS-CoV-2 positive sample pairs, 55.8% were identified as SARS-CoV-2 Omicron BA.1 and 44.2% as Omicron BA.2. Real-time PCR from buccal swabs generated significantly higher quantification cycle (Cq) values compared to those from matched combined oro-/nasopharyngeal swabs and resulted in an increased number of false-negative PCR results. Reduced diagnostic sensitivity of buccal swabs by real-time PCR was observed already at day one after symptom onset. Similarly, antigen test detection rates were reduced in buccal swabs compared to combined oro-/nasopharyngeal swabs.
Conclusion: Our results suggest reduced clinical diagnostic sensitivity of saliva collected using buccal swabs when compared to combined oro-/nasopharyngeal swabs in the detection of SARS-CoV-2 Omicron in symptomatic individuals.
Keywords: B.1.1.529 (Omicron); Buccal swab sampling; Clinical diagnostic sensitivity; SARS-CoV-2; Saliva.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Investigation of saliva, tongue swabs and buccal swabs as alternative specimen types to nasopharyngeal swabs for SARS-CoV-2 testing.J Clin Virol. 2022 Jan;146:105053. doi: 10.1016/j.jcv.2021.105053. Epub 2021 Dec 10. J Clin Virol. 2022. PMID: 34920375 Free PMC article.
-
Omicron Wave SARS-CoV-2 Diagnosis: Evaluation of Saliva, Anterior Nasal, and Nasopharyngeal Swab Samples.Microbiol Spectr. 2022 Dec 21;10(6):e0252122. doi: 10.1128/spectrum.02521-22. Epub 2022 Nov 1. Microbiol Spectr. 2022. PMID: 36318040 Free PMC article.
-
The Sensitivity and Costs of Testing for SARS-CoV-2 Infection With Saliva Versus Nasopharyngeal Swabs : A Systematic Review and Meta-analysis.Ann Intern Med. 2021 Apr;174(4):501-510. doi: 10.7326/M20-6569. Epub 2021 Jan 12. Ann Intern Med. 2021. PMID: 33428446 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
Cited by
-
Impact of Swabbing Location, Self-Swabbing, and Food Intake on SARS-CoV-2 RNA Detection.Microorganisms. 2024 Mar 15;12(3):591. doi: 10.3390/microorganisms12030591. Microorganisms. 2024. PMID: 38543642 Free PMC article.
-
Diagnostic Performance of a Combined Rapid Antigen Test for Detecting SARS-CoV-2, Influenza Virus, and Respiratory Syncytial Virus in Symptomatic Patients in Tertiary Care.J Med Virol. 2025 Jul;97(7):e70493. doi: 10.1002/jmv.70493. J Med Virol. 2025. PMID: 40879159 Free PMC article.
-
Prolonged SARS-CoV-2 Infection in Patients Receiving Anti-CD20 Monoclonal Antibodies: A Diagnostic Challenged by Negative Nasopharyngeal RT-PCR and Successful Treatment with COVID-19 High-Titer Convalescent Plasma.Viruses. 2023 Nov 7;15(11):2220. doi: 10.3390/v15112220. Viruses. 2023. PMID: 38005897 Free PMC article.
-
Accuracy of point-of-care SARS-CoV-2 detection using buccal swabs in pediatric emergency departments.Microbiol Spectr. 2024 Oct 29;12(12):e0188424. doi: 10.1128/spectrum.01884-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 39470284 Free PMC article.
References
-
- RKI. Coronavirus SARS-CoV-2 - Hinweise zur Testung von Patientinnen und Patienten auf SARS-CoV-2, (n.d.). https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Vorl_Testun... (accessed December 6, 2022).
-
- Interim Guidelines for Clinical Specimens for COVID-19 | CDC, (n.d.). https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim... (accessed December 6, 2022).
-
- Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases, (n.d.). https://www.who.int/publications/i/item/10665-331501 (accessed December 6, 2022).
-
- Marais G., Hsiao N., Iranzadeh A., Doolabh D., Enoch A., Chu C., Williamson C., Brink A., Hardie D. Saliva swabs are the preferred sample for Omicron detection. MedRxiv. 2021 doi: 10.1101/2021.12.22.21268246. 2021.12.22.21268246. - DOI
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

