Rejuvenating old fluorophores with new chemistry
- PMID: 37269674
- PMCID: PMC10524207
- DOI: 10.1016/j.cbpa.2023.102335
Rejuvenating old fluorophores with new chemistry
Abstract
The field of organic chemistry began with 19th century scientists identifying and then expanding upon synthetic dye molecules for textiles. In the 20th century, dye chemistry continued with the aim of developing photographic sensitizers and laser dyes. Now, in the 21st century, the rapid evolution of biological imaging techniques provides a new driving force for dye chemistry. Of the extant collection of synthetic fluorescent dyes for biological imaging, two classes reign supreme: rhodamines and cyanines. Here, we provide an overview of recent examples where modern chemistry is used to build these old-but-venerable classes of optically responsive molecules. These new synthetic methods access new fluorophores, which then enable sophisticated imaging experiments leading to new biological insights.
Keywords: Cyanine; Fluorescence; Imaging; Microscopy; Organic chemistry; Rhodamine.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships that may be considered as potential competing interests: Luke Lavis reports a relationship with Eikon Therapeutics that includes consulting or advisory and equity or stocks; patent and patent applications with inventor Luke Lavis are assigned to HHMI.
Figures
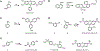
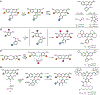
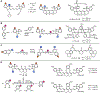
References
-
- Brightman R. Perkin and the dyestuffs industry in Britain. Nature 1956; 177:815.
-
- Einwirkung des Chlorkohlenstoffs auf Anilin. Cyantriphenyldiamin. J. Prakt. Chem 1859; 77:190–191.
-
- Baeyer A. Ueber eine neue Klasse von Farbstoffen. Ber. Dtsch. Chem. Ges 1871; 4:555–558.
-
- Ceresole M. Verfahren zur Darstellung von Farbstoffen aus der Gruppe des Meta-amidophenolphtaleïns. In: Germany: 1887. p. D.R. Patent No. 44002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

