Sleep is required to consolidate odor memory and remodel olfactory synapses
- PMID: 37269832
- PMCID: PMC10354834
- DOI: 10.1016/j.cell.2023.05.006
Sleep is required to consolidate odor memory and remodel olfactory synapses
Abstract
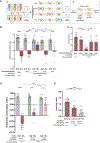
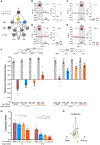
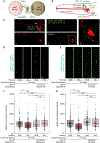
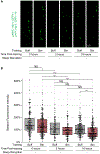
Animals with complex nervous systems demand sleep for memory consolidation and synaptic remodeling. Here, we show that, although the Caenorhabditis elegans nervous system has a limited number of neurons, sleep is necessary for both processes. In addition, it is unclear if, in any system, sleep collaborates with experience to alter synapses between specific neurons and whether this ultimately affects behavior. C. elegans neurons have defined connections and well-described contributions to behavior. We show that spaced odor-training and post-training sleep induce long-term memory. Memory consolidation, but not acquisition, requires a pair of interneurons, the AIYs, which play a role in odor-seeking behavior. In worms that consolidate memory, both sleep and odor conditioning are required to diminish inhibitory synaptic connections between the AWC chemosensory neurons and the AIYs. Thus, we demonstrate in a living organism that sleep is required for events immediately after training that drive memory consolidation and alter synaptic structures.
Keywords: C. elegans; behavior; circuit; memory; memory consolidation; plasticity; single cell; sleep; synapse; systems consolidation.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Comment in
-
Sleep sculpts circuits in every species studied.Cell. 2023 Jun 22;186(13):2730-2732. doi: 10.1016/j.cell.2023.05.042. Cell. 2023. PMID: 37352834
References
-
- Jenkins JG, and Dallenbach KM (1924). Obliviscence during sleep and waking. Am. J. Psychol 35, 605–612. 10.2307/1414040. - DOI
-
- Walker MP, Brakefield T, Morgan A, Hobson JA, and Stickgold R (2002). Practice with sleep makes perfect: sleep-dependent motor skil learning. Neuron 35, 205–211. - PubMed
-
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, and Storm DR (1998). Stimulation of cAMP response element (CRE)-mediate transcription during contextual learning. Nat. Neurosci 1, 595–601. - PubMed
-
- Stubley-Weatherly L, Harding JW, and Wright JW (1996). Effects o discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res. 716, 29–38. - PubMed
Publication types
MeSH terms
Grants and funding
- R35 GM124735/GM/NIGMS NIH HHS/United States
- R24 OD010943/OD/NIH HHS/United States
- F31 NS115572/NS/NINDS NIH HHS/United States
- R15 NS109803/NS/NINDS NIH HHS/United States
- S10 OD028611/OD/NIH HHS/United States
- F31 DC019872/DC/NIDCD NIH HHS/United States
- R01 NS084835/NS/NINDS NIH HHS/United States
- F31 DC014921/DC/NIDCD NIH HHS/United States
- R01 DC005991/DC/NIDCD NIH HHS/United States
- R01 NS087544/NS/NINDS NIH HHS/United States
- S10 RR026758/RR/NCRR NIH HHS/United States
- U24 HG002223/HG/NHGRI NIH HHS/United States
- P40 OD010440/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources

