The role of interleukin-21 in COVID-19 vaccine-induced B cell-mediated immune responses in patients with kidney disease and kidney transplant recipients
- PMID: 37270109
- PMCID: PMC10234364
- DOI: 10.1016/j.ajt.2023.05.025
The role of interleukin-21 in COVID-19 vaccine-induced B cell-mediated immune responses in patients with kidney disease and kidney transplant recipients
Abstract
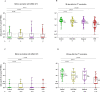
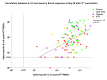
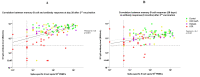
T-cell-mediated help to B cells is required for the development of humoral responses, in which the cytokine interleukin (IL)-21 is key. Here, we studied the mRNA-1273 vaccine-induced SARS-CoV-2-specific memory T-cell IL-21 response, memory B cell response, and immunoglobulin (Ig)G antibody levels in peripheral blood at 28 days after the second vaccination by ELISpot and the fluorescent bead-based multiplex immunoassay, respectively. We included 40 patients with chronic kidney disease (CKD), 34 patients on dialysis, 63 kidney transplant recipients (KTR), and 47 controls. We found that KTR, but not patients with CKD and those receiving dialysis, showed a significantly lower number of SARS-CoV-2-specific IL-21 producing T cells than controls (P < .001). KTR and patients with CKD showed lower numbers of SARS-CoV-2-specific IgG-producing memory B cells when compared with controls (P < .001 and P = .01, respectively). The T-cell IL-21 response was positively associated with the SARS-CoV-2-specific B cell response and the SARS-CoV-2 spike S1-specific IgG antibody levels (both Pearson r = 0.5; P < .001). In addition, SARS-CoV-2-specific B cell responses were shown to be IL-21 dependent. Taken together, we show that IL-21 signaling is important in eliciting robust B cell-mediated immune responses in patients with kidney disease and KTR.
Keywords: COVID-19; SARS-CoV-2; immune responses; interleukin-21; kidney transplant recipients; patients with kidney disease; vaccination.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Kho M.M.L., Reinders M.E.J., Baan C.C., et al. The RECOVAC IR study: the immune response and safety of the mRNA-1273 COVID-19 vaccine in patients with chronic kidney disease, on dialysis or living with a kidney transplant. Nephrol Dial Transplant. 2021;36(9):1761–1764. doi: 10.1093/ndt/gfab186. - DOI - PMC - PubMed
-
- Sanders J.F., Messchendorp A.L., de Vries R.D., et al. Antibody and T-cell responses 6 months after COVID-19 mRNA-1273 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Clin Infect Dis. 2023;76(3):e188–e199. doi: 10.1093/cid/ciac557. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

