Voxelotor does not inhibit sickle hemoglobin fiber formation upon complete deoxygenation
- PMID: 37270670
- PMCID: PMC10397806
- DOI: 10.1016/j.bpj.2023.05.034
Voxelotor does not inhibit sickle hemoglobin fiber formation upon complete deoxygenation
Abstract
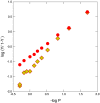
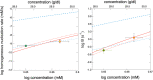
The drug voxelotor (commercially known as Oxbryta) has been approved by the US Food and Drug Administration for the treatment of sickle cell disease. It is known to reduce disease-causing sickling by inhibiting the transformation of the non-polymerizing, high-oxygen-affinity R quaternary structure of sickle hemoglobin into its polymerizing, low-affinity T quaternary structure. It has not been established whether the binding of the drug has anti-sickling effects beyond restricting the change of quaternary structure. By using a laser photolysis method that employs microscope optics, we have determined that fully deoxygenated sickle hemoglobin will assume the T structure. We show that the nucleation rates essential to generate the sickle fibers are not significantly affected by voxelotor. The method employed here should be useful for determining the mechanism of sickling inhibition for proposed drugs.
Copyright © 2023 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Pauling L., Itano H., et al. Wells I.C. Sickle cell anemia, a molecular disease. Science. 1949;110:543–548. - PubMed
-
- Perutz M.F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–739. http://www.ncbi.nlm.nih.gov/pubmed/5528785 - PubMed
-
- Gelin B.R., Lee A.W., Karplus M. Hemoglobin tertiary structural change on ligand binding. Its role in the co-operative mechanism. J. Mol. Biol. 1983;171:489–559. doi: 10.1016/0022-2836(83)90042-6. https://www.ncbi.nlm.nih.gov/pubmed/6663623 - DOI - PubMed
-
- Padlan E.A., Love W.E. Refined Crystal Structure of Deoxyhemoglobin S I. Restrained lest squares refinement at 3.0 A resolution. J. Biol. Chem. 1985;260:8272–8279. - PubMed
-
- Sunshine H.R., Hofrichter J., et al. Eaton W.A. Oxygen binding by sickle cell hemoglobin polymers. J. Mol. Biol. 1982;158:251–273. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

