Patient-Reported Outcomes During and After Treatment for Locally Advanced Rectal Cancer in the PROSPECT Trial (Alliance N1048)
- PMID: 37270691
- PMCID: PMC10351948
- DOI: 10.1200/JCO.23.00903
Patient-Reported Outcomes During and After Treatment for Locally Advanced Rectal Cancer in the PROSPECT Trial (Alliance N1048)
Abstract
Purpose: The standard of care for locally advanced rectal cancer in North America is neoadjuvant pelvic chemoradiation with fluorouracil (5FUCRT). Neoadjuvant chemotherapy with fluorouracil and oxaliplatin (FOLFOX) is an alternative that may spare patients the morbidity of radiation. Understanding the relative patient experiences with these options is necessary to inform treatment decisions.
Methods: PROSPECT was a multicenter, unblinded, noninferiority, randomized trial of neoadjuvant FOLFOX versus 5FUCRT, which enrolled adults with rectal cancer clinically staged as T2N+, cT3N-, or cT3N+ who were candidates for sphincter-sparing surgery. Neoadjuvant FOLFOX was given in six cycles over 12 weeks, followed by surgery. Neoadjuvant 5FUCRT was delivered in 28 fractions over 5.5 weeks, followed by surgery. Adjuvant chemotherapy was suggested but not mandated in both groups. Enrolled patients were asked to provide patient-reported outcomes (PROs) at baseline, during neoadjuvant treatment, and at 12 months after surgery. PROs included 14 symptoms from the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Additional PRO instruments measured bowel, bladder, sexual function, and health-related quality of life (HRQL).
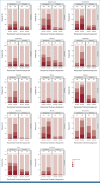
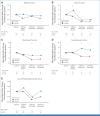
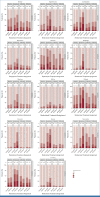
Results: From June 2012 to December 2018, 1,194 patients were randomly assigned, 1,128 initiated treatment, and 940 contributed PRO-CTCAE data (493 FOLFOX; 447 5FUCRT). During neoadjuvant treatment, patients reported significantly lower rates of diarrhea and better overall bowel function with FOLFOX while anxiety, appetite loss, constipation, depression, dysphagia, dyspnea, edema, fatigue, mucositis, nausea, neuropathy, and vomiting were lower with 5FUCRT (all multiplicity adjusted P < .05). At 12 months after surgery, patients randomly assigned to FOLFOX reported significantly lower rates of fatigue and neuropathy and better sexual function versus 5FUCRT (all multiplicity adjusted P < .05). Neither bladder function nor HRQL differed between groups at any time point.
Conclusion: For patients with locally advanced rectal cancer choosing between neoadjuvant FOLFOX and 5FUCRT, the distinctive PRO profiles inform treatment selection and shared decision making.
Trial registration: ClinicalTrials.gov NCT01515787.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- Henry DH, Viswanathan HN, Elkin EP, et al. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791–801. - PubMed
-
- Carlotto A, Hogsett VL, Maiorini EM, et al. The economic burden of toxicities associated with cancer treatment: Review of the literature and analysis of nausea and vomiting, diarrhoea, oral mucositis and fatigue. Pharmacoeconomics. 2013;31:753–766. - PubMed
-
- U.S. Food & Drug Administration, Oncology Center of Excellence, Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research https://www.fda.gov/media/149994/download Guidance document: Core patient-reported outcomes in cancer clinical trials, draft guidance for industry, 2021.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U01 CA233046/CA/NCI NIH HHS/United States
- UG1 CA233373/CA/NCI NIH HHS/United States
- HHSN261200800063C/CA/NCI NIH HHS/United States
- UG1 CA189823/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

