Tepotinib Treatment in Patients With MET Exon 14-Skipping Non-Small Cell Lung Cancer: Long-term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial
- PMID: 37270698
- PMCID: PMC10240398
- DOI: 10.1001/jamaoncol.2023.1962
Tepotinib Treatment in Patients With MET Exon 14-Skipping Non-Small Cell Lung Cancer: Long-term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial
Erratum in
-
Errors in the Conflicts of Interest Section, Figure 2, and Affiliations.JAMA Oncol. 2023 Sep 1;9(9):1300. doi: 10.1001/jamaoncol.2023.2810. JAMA Oncol. 2023. PMID: 37471093 Free PMC article. No abstract available.
Abstract
Importance: MET inhibitors have recently demonstrated clinical activity in patients with MET exon 14 (METex14)-skipping non-small cell lung cancer (NSCLC); however, data with longer follow-up and in larger populations are needed to further optimize therapeutic approaches.
Objective: To assess the long-term efficacy and safety of tepotinib, a potent and highly selective MET inhibitor, in patients with METex14-skipping NSCLC in the VISION study.
Design, setting, and participants: The VISION phase 2 nonrandomized clinical trial was a multicohort, open-label, multicenter study that enrolled patients with METex14-skipping advanced/metastatic NSCLC (cohorts A and C) from September 2016 to May 2021. Cohort C (>18 months' follow-up) was an independent cohort, designed to confirm findings from cohort A (>35 months' follow-up). Data cutoff was November 20, 2022.
Intervention: Patients received tepotinib, 500 mg (450 mg active moiety), once daily.
Main outcomes and measures: The primary end point was objective response by independent review committee (RECIST v1.1). Secondary end points included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
Results: Cohorts A and C included 313 patients (50.8% female, 33.9% Asian; median [range] age, 72 [41-94] years). The objective response rate (ORR) was 51.4% (95% CI, 45.8%-57.1%) with a median (m)DOR of 18.0 (95% CI, 12.4-46.4) months. In cohort C (n = 161), an ORR of 55.9% (95% CI, 47.9%-63.7%) with an mDOR of 20.8 (95% CI, 12.6-not estimable [NE]) months was reported across treatment lines, comparable to cohort A (n = 152). In treatment-naive patients (cohorts A and C; n = 164), ORR was 57.3% (95% CI, 49.4%-65.0%) and mDOR was 46.4 (95% CI, 13.8-NE) months. In previously treated patients (n = 149), ORR was 45.0% (95% CI, 36.8%-53.3%) and mDOR was 12.6 (95% CI, 9.5-18.5) months. Peripheral edema, the most common treatment-related adverse event, occurred in 210 patients (67.1%) (35 [11.2%] experienced grade ≥3 events).
Conclusions and relevance: The findings from cohort C in this nonrandomized clinical trial supported the results from original cohort A. Overall, the long-term outcomes of VISION demonstrated robust and durable clinical activity following treatment with tepotinib, particularly in the treatment-naive setting, in the largest known clinical trial of patients with METex14-skipping NSCLC, supporting the global approvals of tepotinib and enabling clinicians to implement this therapeutic approach for such patients.
Trial registration: ClinicalTrials.gov Identifier: NCT02864992.
Conflict of interest statement
Figures
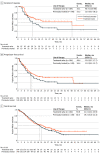

Comment in
-
MET targeted therapy in non-small cell lung cancer patients with MET exon 14-skipping mutations.Transl Lung Cancer Res. 2024 Apr 29;13(4):940-946. doi: 10.21037/tlcr-24-98. Epub 2024 Apr 25. Transl Lung Cancer Res. 2024. PMID: 38736494 Free PMC article. No abstract available.
-
Diagnosis and treatment of non-small cell lung cancer (NSCLC) harboring MET Ex14 skipping: have we met the desired drug?Transl Lung Cancer Res. 2024 Jun 30;13(6):1438-1443. doi: 10.21037/tlcr-24-93. Epub 2024 Jun 14. Transl Lung Cancer Res. 2024. PMID: 38973959 Free PMC article. No abstract available.
References
-
- Viteri S, Mazieres J, Veillon R, et al. . 1286P Activity of tepotinib in brain metastases (BM): preclinical models and clinical data from patients (pts) with MET exon 14 (METex14) skipping NSCLC. Ann Oncol. 2020;31(suppl 4):S754-S840. doi:10.1016/j.annonc.2020.08.1600 - DOI
-
- Thomas M, Garassino MC, Felip E, et al. . Tepotinib in patients with MET exon 14 (METex14) skipping NSCLC: primary analysis of the confirmatory VISION cohort C. J Thorac Oncol. 2022;17(9):S9-S10.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

