Association Between Duration of Immunotherapy and Overall Survival in Advanced Non-Small Cell Lung Cancer
- PMID: 37270700
- PMCID: PMC10240399
- DOI: 10.1001/jamaoncol.2023.1891
Association Between Duration of Immunotherapy and Overall Survival in Advanced Non-Small Cell Lung Cancer
Abstract
Importance: For patients with advanced non-small cell lung cancer (NSCLC) treated with frontline immunotherapy-based treatment, the optimal duration of immune checkpoint inhibitor (ICI) treatment is unknown.
Objective: To assess practice patterns surrounding ICI treatment discontinuation at 2 years and to evaluate the association of duration of therapy with overall survival in patients who received fixed-duration ICI therapy for 2 years vs those who continued therapy beyond 2 years.
Design, setting, and participants: This retrospective, population-based cohort study included adult patients in a clinical database diagnosed with advanced NSCLC from 2016 to 2020, who received frontline immunotherapy-based treatment. The data cutoff was August 31, 2022; data analysis was conducted from October 2022 to January 2023.
Exposures: Treatment discontinuation at 2 years (between 700 and 760 days, fixed duration) vs continued treatment beyond 2 years (greater than 760 days, indefinite duration).
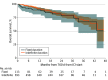
Main outcomes and measures: Overall survival from 760 days was analyzed using Kaplan-Meier methods. Multivariable Cox regression that adjusted for patient-specific and cancer-specific factors was used to compare survival beyond 760 days between the fixed-duration group and the indefinite-duration group.
Results: Of 1091 patients in the analytic cohort who were still on ICI treatment at 2 years after exclusion criteria for death and progression were applied, 113 patients (median [IQR] age, 69 [62-75] years; 62 [54.9%] female; 86 [76.1%] White) were in the fixed-duration group, and 593 patients (median [IQR] age, 69 [62-76] years; 282 [47.6%] female; 414 [69.8%] White) were in the indefinite-duration group. Patients in the fixed-duration group were more likely to have a history of smoking (99% vs 93%; P = .01) and be treated at an academic center (22% vs 11%; P = .001). Two-year overall survival from 760 days was 79% (95% CI, 66%-87%) in the fixed-duration group and 81% (95% CI, 77%-85%) in the indefinite-duration group. There was no statistically significant difference in overall survival between patients in the fixed-duration and indefinite-duration groups, either on univariate (hazard ratio [HR] 1.26; 95% CI, 0.77-2.08; P = .36) or multivariable (HR 1.33; 95% CI, 0.78-2.25; P = .29) Cox regression. Approximately 1 in 5 patients discontinued immunotherapy at 2 years in the absence of progression.
Conclusions and relevance: In a retrospective clinical cohort of patients with advanced NSCLC who were treated with immunotherapy and were progression-free at 2 years, approximately only 1 in 5 discontinued treatment. The lack of statistically significant overall survival advantage for the indefinite-duration cohort on adjusted analysis provides reassurance to patients and clinicians who wish to discontinue immunotherapy at 2 years.
Conflict of interest statement
Figures


Comment on
-
Clinical Decision-Making in the Real World-The Perfect as the Enemy of the Good.JAMA Oncol. 2023 Aug 1;9(8):1082. doi: 10.1001/jamaoncol.2023.1811. JAMA Oncol. 2023. PMID: 37270699 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

