Maresin 1 activates brown adipose tissue and promotes browning of white adipose tissue in mice
- PMID: 37271337
- PMCID: PMC10331312
- DOI: 10.1016/j.molmet.2023.101749
Maresin 1 activates brown adipose tissue and promotes browning of white adipose tissue in mice
Abstract
Objective: Maresin 1 (MaR1) is a docosahexaenoic acid-derived proresolving lipid mediator with insulin-sensitizing and anti-steatosis properties. Here, we aim to unravel MaR1 actions on brown adipose tissue (BAT) activation and white adipose tissue (WAT) browning.
Methods: MaR1 actions were tested in cultured murine brown adipocytes and in human mesenchymal stem cells (hMSC)-derived adipocytes. In vivo effects of MaR1 were tested in diet-induced obese (DIO) mice and lean WT and Il6 knockout (Il6-/-) mice.
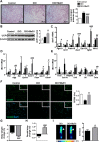
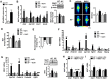
Results: In cultured differentiated murine brown adipocytes, MaR1 reduces the expression of inflammatory genes, while stimulates glucose uptake, fatty acid utilization and oxygen consumption rate, along with the upregulation of mitochondrial mass and genes involved in mitochondrial biogenesis and function and the thermogenic program. In Leucine Rich Repeat Containing G Protein-Coupled Receptor 6 (LGR6)-depleted brown adipocytes using siRNA, the stimulatory effect of MaR1 on thermogenic genes was abrogated. In DIO mice, MaR1 promotes BAT remodeling, characterized by higher expression of genes encoding for master regulators of mitochondrial biogenesis and function and iBAT thermogenic activation, together with increased M2 macrophage markers. In addition, MaR1-treated DIO mice exhibit a better response to cold-induced BAT activation. Moreover, MaR1 induces a beige adipocyte signature in inguinal WAT of DIO mice and in hMSC-derived adipocytes. MaR1 potentiates Il6 expression in brown adipocytes and BAT of cold exposed lean WT mice. Interestingly, the thermogenic properties of MaR1 were abrogated in Il6-/- mice.
Conclusions: These data reveal MaR1 as a novel agent that promotes BAT activation and WAT browning by regulating thermogenic program in adipocytes and M2 polarization of macrophages. Moreover, our data suggest that LGR6 receptor is mediating MaR1 actions on brown adipocytes, and that IL-6 is required for the thermogenic effects of MaR1.
Keywords: Brown/beige adipose tissue; Interleukin-6; LGR6; Maresin 1; Obesity.
Copyright © 2023 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

