Meta-analysis of the efficacy and safety of sevelamer as hyperphosphatemia therapy for hemodialysis patients
- PMID: 37272189
- PMCID: PMC10243412
- DOI: 10.1080/0886022X.2023.2210230
Meta-analysis of the efficacy and safety of sevelamer as hyperphosphatemia therapy for hemodialysis patients
Abstract
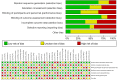
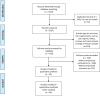
This study was designed to examine the relative safety and efficacy of sevelamer in the treatment of chronic kidney disease (CKD) patients in comparison to placebo, calcium carbonate (CC), or lanthanum carbonate (LC). The PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI) databases were searched for articles published through 18 June 2022. The quality of relevant studies was independently analyzed by two investigators who also extracted data from these manuscripts as per Cochrane Collaboration Handbook 5.3. The safety and efficacy of sevelamer as a treatment for hyperphosphatemia in CKD patients were then examined through a meta-analysis, with the primary patient-level outcomes of interest in this analysis being all-cause mortality and the incidence of gastrointestinal adverse effects. Vascular calcification score was also examined as an intermediate outcome, while serum biochemical parameters including levels of phosphate (P), calcium (Ca), intact parathyroid hormone (iPTH), lipids, C-reactive protein (CRP), or fibroblast growth factor-23 (FGF-23) were additionally assessed. In total, this meta-analysis incorporated data from 34 randomized controlled trials (RCTs) enrolling 2802 patients. Sevelamer was associated with reduced all-cause mortality (RR 0.28, CI 0.19 - 0.41, very low certainty) and Vessel calcification score (RR -0.58, CI -1.11 to -0.04, low certainty) and induced less hypercalcemia (MD -0.28, CI 0.40 to -0.16, low certainty) and hyperphosphatemia (MD -0.22, CI -0.32 to -0.13, low certainty) when compared with Ca-based binders in CKD5D individuals. No significant differences in gastrointestinal adverse events (GAEs) incidence were observed. These data suggest that sevelamer may represent a beneficial means of protecting CKD patients against death and vessel calcification when used to treat hyperphosphatemia, while we found no clinically important benefits in decreasing gastrointestinal adverse effects.
Keywords: Sevelamer; chronic kidney disease; hyperphosphatemia; meta-analysis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures









Similar articles
-
Sevelamer Versus Calcium-Based Binders for Treatment of Hyperphosphatemia in CKD: A Meta-Analysis of Randomized Controlled Trials.Clin J Am Soc Nephrol. 2016 Feb 5;11(2):232-44. doi: 10.2215/CJN.06800615. Epub 2015 Dec 14. Clin J Am Soc Nephrol. 2016. PMID: 26668024 Free PMC article. Review.
-
Safety and effectiveness of lanthanum carbonate for hyperphosphatemia in chronic kidney disease (CKD) patients: a meta-analysis.Ren Fail. 2021 Dec;43(1):1378-1393. doi: 10.1080/0886022X.2021.1986068. Ren Fail. 2021. PMID: 34602015 Free PMC article. Review.
-
Progress in pharmacotherapy for the treatment of hyperphosphatemia in renal failure.Expert Opin Pharmacother. 2023 Sep-Dec;24(15):1737-1746. doi: 10.1080/14656566.2023.2243817. Epub 2023 Aug 11. Expert Opin Pharmacother. 2023. PMID: 37527180 Review.
-
Efficacy and Safety of Ferric Citrate on Hyperphosphatemia among Chinese Patients with Chronic Kidney Disease Undergoing Hemodialysis: A Phase III Multicenter Randomized Open-Label Active-Drug-Controlled Study.Am J Nephrol. 2023;54(11-12):479-488. doi: 10.1159/000534484. Epub 2023 Oct 9. Am J Nephrol. 2023. PMID: 37812931 Clinical Trial.
-
Initiation of Sevelamer and Mortality among Hemodialysis Patients Treated with Calcium-Based Phosphate Binders.Clin J Am Soc Nephrol. 2017 Sep 7;12(9):1489-1497. doi: 10.2215/CJN.13091216. Epub 2017 Jul 19. Clin J Am Soc Nephrol. 2017. PMID: 28724618 Free PMC article.
Cited by
-
Individualizing the lifesaving journey for calciphylaxis: addressing rapidly progressive attacks with multidimensional and AI research for regenerative medicine.Ren Fail. 2024 Dec;46(2):2392846. doi: 10.1080/0886022X.2024.2392846. Epub 2024 Sep 5. Ren Fail. 2024. PMID: 39234636 Free PMC article. No abstract available.
-
Multifaceted skeletal effects of sevelamer carbonate in a secondary hyperparathyroidism model.Endocrine. 2025 May;88(2):581-596. doi: 10.1007/s12020-025-04180-4. Epub 2025 Feb 7. Endocrine. 2025. PMID: 39918636
-
Gut microbiome remodeling in chronic kidney disease: implications of kidney replacement therapies and therapeutic interventions.Front Med (Lausanne). 2025 Jul 15;12:1620247. doi: 10.3389/fmed.2025.1620247. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40735439 Free PMC article. Review.
-
Therapeutic effectiveness and safety profile of lanthanum carbonate in conjunction with calcium carbonate for managing hyperphosphatemia in hemodialysis patients: a meta-analysis of randomized controlled trials.Am J Transl Res. 2024 Nov 15;16(11):6980-6990. doi: 10.62347/WXBL2590. eCollection 2024. Am J Transl Res. 2024. PMID: 39678559 Free PMC article. Review.
References
-
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. - PubMed
-
- Moe SM, Chen NX.. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19(2):213–216. - PubMed
-
- Basutkar RS, Varghese R, Mathew NK, et al. . Systematic review and meta-analysis of potential pleiotropic effects of sevelamer in chronic kidney disease: beyond phosphate control. Nephrology (Carlton). 2022;27(4):337–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
