Insufficient GDF15 expression predisposes women to unexplained recurrent pregnancy loss by impairing extravillous trophoblast invasion
- PMID: 37272232
- PMCID: PMC10693185
- DOI: 10.1111/cpr.13514
Insufficient GDF15 expression predisposes women to unexplained recurrent pregnancy loss by impairing extravillous trophoblast invasion
Abstract
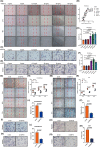
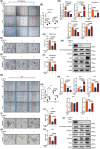
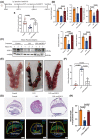
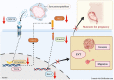
Insufficient extravillous trophoblast (EVT) invasion during early placentation has been shown to contribute to recurrent pregnancy loss (RPL). However, the regulatory factors involved and their involvement in RPL pathogenesis remain unknown. Here, we found aberrantly decreased growth differentiation factor 15 (GDF15) levels in both first-trimester villous and serum samples of unexplained recurrent pregnancy loss (URPL) patients as compared with normal pregnancies. Moreover, GDF15 knockdown significantly reduced the invasiveness of both HTR-8/SVneo cells and primary human EVT cells and suppressed the Jagged-1 (JAG1)/NOTCH3/HES1 pathway activity, and JAG1 overexpression rescued the invasion phenotype of the GDF15 knockdown cells. Induction of a lipopolysaccharide-induced abortion model in mice resulted in significantly reduced GDF15 level in the placenta and serum, as well as increased rates of embryonic resorption, and these effects were reversed by administration of recombinant GDF15. Our study thus demonstrates that insufficient GDF15 level at the first-trimester maternal-foetal interface contribute to the pathogenesis of URPL by impairing EVT invasion and suppressing JAG1/NOTCH3/HES1 pathway activity, and suggests that supplementation with GDF15 could benefit early pregnancy maintenance and reduce the risk of early pregnancy.
© 2023 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures



References
MeSH terms
Substances
Grants and funding
- 2022YFC2702400/National Key Research and Development Program of China
- 31988101/the National Natural Science Foundation of China
- 82101784/the National Natural Science Foundation of China
- 82171648/the National Natural Science Foundation of China
- 2021LCZX02/Key Research and Development Program of Shandong Province
LinkOut - more resources
Full Text Sources
Miscellaneous

