A Programmable CRISPR/Cas9 Toolkit Improves Lycopene Production in Bacillus subtilis
- PMID: 37272803
- PMCID: PMC10305015
- DOI: 10.1128/aem.00230-23
A Programmable CRISPR/Cas9 Toolkit Improves Lycopene Production in Bacillus subtilis
Abstract
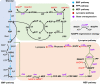
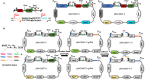
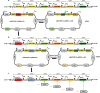
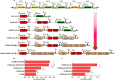
Bacillus subtilis has been widely used and generally recognized as a safe host for the production of recombinant proteins, high-value chemicals, and pharmaceuticals. Thus, its metabolic engineering attracts significant attention. Nevertheless, the limited availability of selective markers makes this process difficult and time-consuming, especially in the case of multistep biosynthetic pathways. Here, we employ CRISPR/Cas9 technology to build an easy cloning toolkit that addresses commonly encountered obstacles in the metabolic engineering of B. subtilis, including the chromosomal integration locus, promoter, terminator, and guide RNA (gRNA) target. Six promoters were characterized, and the promoter strengths ranged from 0.9- to 23-fold that of the commonly used strong promoter P43. We characterized seven terminators in B. subtilis, and the termination efficiencies (TEs) of the seven terminators are all more than 90%. Six gRNA targets were designed upstream of the promoter and downstream of the terminator. Using a green fluorescent protein (GFP) reporter, we confirmed integration efficiency with the single-locus integration site is up to 100%. We demonstrated the applicability of this toolkit by optimizing the expression of a challenging but industrially important product, lycopene. By heterologous expression of the essential genes for lycopene synthesis on the B. subtilis genome, a total of 13 key genes involved in the lycopene biosynthetic pathway were manipulated. Moreover, our findings showed that the gene cluster ispG-idi-dxs-ispD could positively affect the production of lycopene, while the cluster dxr-ispE-ispF-ispH had a negative effect on lycopene production. Hence, our multilocus integration strategy can facilitate the pathway assembly for production of complex chemicals and pharmaceuticals in B. subtilis. IMPORTANCE We present a toolkit that allows for rapid cloning procedures and one-step subcloning to move from plasmid-based expression to stable chromosome integration and expression in a production strain in less than a week. The utility of the customized tool was demonstrated by integrating the MEP (2C-methyl-d-erythritol-4-phosphate) pathway, part of the pentose phosphate pathway (PPP), and the hetero-lycopene biosynthesis genes by stable expression in the genome. The tool could be useful to engineer B. subtilis strains through diverse recombination events and ultimately improve its potential and scope of industrial application as biological chassis.
Keywords: Bacillus subtilis; CRISPR; MEP pathway; genome editing; lycopene; microbial fermentation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Liu L, Liu Y, Shin HD, Chen RR, Wang NS, Li J, Du G, Chen J. 2013. Developing Bacillus spp. as a cell factory for production of microbial enzymes and industrially important biochemicals in the context of systems and synthetic biology. Appl Microbiol Biotechnol 97:6113–6127. doi: 10.1007/s00253-013-4960-4. - DOI - PubMed
-
- Ou MS, Mohammed N, Ingram LO, Shanmugam KT. 2009. Thermophilic Bacillus coagulans requires less cellulases for simultaneous saccharification and fermentation of cellulose to products than mesophilic microbial biocatalysts. Appl Biochem Biotechnol 155:379–385. doi: 10.1007/s12010-008-8509-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

