Extracellular vesicles from human plasma dampen inflammation and promote tissue repair functions in macrophages
- PMID: 37272889
- PMCID: PMC10241174
- DOI: 10.1002/jev2.12331
Extracellular vesicles from human plasma dampen inflammation and promote tissue repair functions in macrophages
Abstract
Although inflammation is a vital defence response to infection, if left uncontrolled, it can lead to pathology. Macrophages are critical players both in driving the inflammatory response and in the subsequent events required for restoring tissue homeostasis. Extracellular vesicles (EVs) are membrane-enclosed structures released by cells that mediate intercellular communication and are present in all biological fluids, including blood. Herein, we show that extracellular vesicles from plasma (pEVs) play a relevant role in the control of inflammation by counteracting PAMP-induced macrophage activation. Indeed, pEV-treatment of macrophages simultaneously with or prior to PAMP exposure reduced the secretion of pro-inflammatory IL-6 and TNF-α and increased IL-10 response. This anti-inflammatory activity was associated with the promotion of tissue-repair functions in macrophages, characterized by augmented efferocytosis and pro-angiogenic capacity, and increased expression of VEGFa, CD300e, RGS2 and CD93, genes involved in cell growth and tissue remodelling. We also show that simultaneous stimulation of macrophages with a PAMP and pEVs promoted COX2 expression and CREB phosphorylation as well as the accumulation of higher concentrations of PGE2 in cell culture supernatants. Remarkably, the anti-inflammatory activity of pEVs was abolished if cells were treated with a pharmacological inhibitor of COX2, indicating that pEV-mediated induction of COX2 is critical for the pEV-mediated inhibition of inflammation. Finally, we show that pEVs added to monocytes prior to their M-CSF-induced differentiation to macrophages increased efferocytosis and diminished pro-inflammatory cytokine responses to PAMP stimulation. In conclusion, our results suggest that pEVs are endogenous homeostatic modulators of macrophages, activating the PGE2/CREB pathway, decreasing the production of inflammatory cytokines and promoting tissue repair functions.
Keywords: CREB; PGE2; exosomes; extracellular vesicles; human plasma; infection; inflammation; macrophages; resolution; tissue homeostasis; wound-healing.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

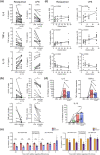
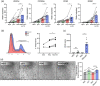
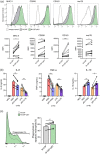

References
-
- Atay, S. , Gercel‐Taylor, C. , & Taylor, D. D. (2011). Human trophoblast‐derived exosomal fibronectin induces pro‐inflammatory IL‐1β production by macrophages. American Journal of Reproductive Immunology, 66(4), 259–269. - PubMed
-
- Baluk, P. , Hirata, A. , Thurston, G. , Fujiwara, T. , Neal, C. R. , Michel, C. C. , & McDonald, D. M. (1997). Endothelial gaps: Time course of formation and closure in inflamed venules of rats. American Journal of Physiology, 272(1 Pt 1), L155–L170. - PubMed
-
- Bannoud, N. , García, P. A. , Gambarte‐Tudela, J. , Sundblad, V. , Cagnoni, A. J. , Bach, C. A. , Pérez Saez, J. M. , Blidner, A. G. , Maller, S. M. , Mariño, K. V. , Salatino, M. , Cerliani, J. P. , Rabinovich, G. A. , & Croci, D. O. (2022). Untangling galectin‐mediated circuits that control hypoxia‐driven angiogenesis. Methods in Molecular Biology, 2442, 635–653. 10.1007/978-1-0716-2055-7_34 - DOI - PubMed
-
- Barrès, C. , Blanc, L. , Bette‐Bobillo, P. , André, S. , Mamoun, R. , Gabius, H.‐J. , & Vidal, M. (2010). Galectin‐5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood, 115(3), 696–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

