Extracellular vesicles enhance pulmonary transduction of stably associated adeno-associated virus following intratracheal administration
- PMID: 37272896
- PMCID: PMC10241173
- DOI: 10.1002/jev2.12324
Extracellular vesicles enhance pulmonary transduction of stably associated adeno-associated virus following intratracheal administration
Abstract
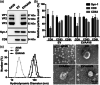
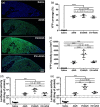
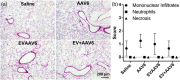
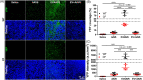
Adeno-associated virus (AAV) vector has shown multiple clinical breakthroughs, but its clinical implementation in inhaled gene therapy remains elusive due to difficulty in transducing lung airway cells. We demonstrate here AAV serotype 6 (AAV6) associated with extracellular vesicles (EVs) and secreted from vector-producing HEK-293 cells during vector preparation (EVAAV6) as a safe and highly efficacious gene delivery platform for inhaled gene therapy applications. Specifically, we discovered that EVAAV6 provided markedly enhanced reporter transgene expression in mucus-covered air-liquid interface (ALI) cultures of primary human bronchial and nasal epithelial cells as well as in mouse lung airways compared to standard preparations of AAV6 alone. Of note, AAV6 has been previously shown to outperform other clinically tested AAV serotypes, including those approved by the FDA for treating non-lung diseases, in transducing ALI cultures of primary human airway cells. We provide compelling experimental evidence that the superior performance of EVAAV6 is attributed to the ability of EV to facilitate mucus penetration and cellular entry/transduction of AAV6. The tight and stable linkage between AAV6 and EVs appears essential to exploit the benefits of EVs given that a physical mixture of individually prepared EVs and AAV6 failed to mediate EV-AAV6 interactions or to enhance gene transfer efficacy.
Keywords: Extracellular vesicles; adeno-associated virus; airway mucus; cellular entry; inhaled gene therapy.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
There is no conflict of interest to be declared by the authors.
Figures

References
-
- Adewale, A. T. , Falk Libby, E. , Fu, L. , Lenzie, A. , Boitet, E. R. , Birket, S E. , Petty, C. F. , Johns, J. D , Mazur, M. , Tearney, G. J. , Copeland, D. , Durham, C. , & Rowe, S. M. (2020). Novel therapy of bicarbonate, glutathione, and ascorbic acid improves cystic fibrosis mucus transport. American Journal of Respiratory Cell and Molecular Biology, 63(3), p. 362–373. - PMC - PubMed
-
- Aslanidi, G. V. , Rivers, A. E. , Ortiz, L. , Song, L. , Ling, C. , Govindasamy, L. , Van Vliet, K. , Tan, M. , Agbandje‐Mckenna, M. , & Srivastava, A. (2013). Optimization of the capsid of recombinant adeno‐associated virus 2 (AAV2) vectors: The final threshold. PLoS One, 8(3), p. e59142. - PMC - PubMed
-
- Boutin, S. , Monteilhet, V. , Veron, P. , Leborgne, C. , Benveniste, O. , Montus, M. F. , & Masurier, C. (2010). Prevalence of serum IgG and neutralizing factors against adeno‐associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Human Gene Therapy, 21(6), p. 704–712. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

