Unsupervised [18F]Flortaucipir cutoffs for tau positivity and staging in Alzheimer's disease
- PMID: 37272955
- PMCID: PMC10542510
- DOI: 10.1007/s00259-023-06280-7
Unsupervised [18F]Flortaucipir cutoffs for tau positivity and staging in Alzheimer's disease
Abstract
Purpose: Several [18F]Flortaucipir cutoffs have been proposed for tau PET positivity (T+) in Alzheimer's disease (AD), but none were data-driven. The aim of this study was to establish and validate unsupervised T+ cutoffs by applying Gaussian mixture models (GMM).
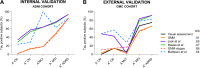
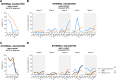
Methods: Amyloid negative (A-) cognitively normal (CN) and amyloid positive (A+) AD-related dementia (ADRD) subjects from ADNI (n=269) were included. ADNI (n=475) and Geneva Memory Clinic (GMC) cohorts (n=98) were used for validation. GMM-based cutoffs were extracted for the temporal meta-ROI, and validated against previously published cutoffs and visual rating.
Results: GMM-based cutoffs classified less subjects as T+, mainly in the A- CN (<3.4% vs >28.5%) and A+ CN (<14.5% vs >42.9%) groups and showed higher agreement with visual rating (ICC=0.91 vs ICC<0.62) than published cutoffs.
Conclusion: We provided reliable data-driven [18F]Flortaucipir cutoffs for in vivo T+ detection in AD. These cutoffs might be useful to select participants in clinical and research studies.
Keywords: Alzheimer’s disease; Cutoff; Gaussian mixture model; Tau PET; Tau positivity; [18F]Flortaucipir.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hanseeuw BJ, Betensky RA, Jacobs HI, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 2019;76:915–924. doi: 10.1001/jamaneurol.2019.1424. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

