Targeted imaging of very late antigen-4 for noninvasive assessment of lung inflammation-fibrosis axis
- PMID: 37273103
- PMCID: PMC10240482
- DOI: 10.1186/s13550-023-01006-0
Targeted imaging of very late antigen-4 for noninvasive assessment of lung inflammation-fibrosis axis
Abstract
Background: The lack of noninvasive methods for assessment of dysregulated inflammation as a major driver of fibrosis (i.e., inflammation-fibrosis axis) has been a major challenge to precision management of fibrotic lung diseases. Here, we determined the potential of very late antigen-4 (VLA-4)-targeted positron emission tomography (PET) to detect inflammation in a mouse model of bleomycin-induced fibrotic lung injury.
Method: Single time-point and longitudinal VLA-4-targeted PET was performed using a high-affinity peptidomimetic radiotracer, 64Cu-LLP2A, at weeks 1, 2, and 4 after bleomycin-induced (2.5 units/kg) lung injury in C57BL/6J mice. The severity of fibrosis was determined by measuring the hydroxyproline content of the lungs and expression of markers of extracellular matrix remodeling. Flow cytometry and histology was performed to determine VLA-4 expression across different leukocyte subsets and their spatial distribution.
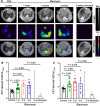
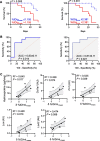
Results: Lung uptake of 64Cu-LLP2A was significantly elevated throughout different stages of the progression of bleomycin-induced injury. High lung uptake of 64Cu-LLP2A at week-1 post-bleomycin was a predictor of poor survival over the 4-week follow up, supporting the prognostic potential of 64Cu-LLP2A PET during the early stage of the disease. Additionally, the progressive increase in 64Cu-LLP2A uptake from week-1 to week-4 post-bleomycin correlated with the ultimate extent of lung fibrosis and ECM remodeling. Flow cytometry revealed that LLP2A binding was restricted to leukocytes. A combination of increased expression of VLA-4 by alveolar macrophages and accumulation of VLA-4-expressing interstitial and monocyte-derived macrophages as well as dendritic cells was noted in bleomycin-injured, compared to control, lungs. Histology confirmed the increased expression of VLA-4 in bleomycin-injured lungs, particularly in inflamed and fibrotic regions.
Conclusions: VLA-4-targeted PET allows for assessment of the inflammation-fibrosis axis and prediction of disease progression in a murine model. The potential of 64Cu-LLP2A PET for assessment of the inflammation-fibrosis axis in human fibrotic lung diseases needs to be further investigated.
Keywords: Inflammation; Lung fibrosis; Molecular imaging; PET; Very late antigen-4.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures



Similar articles
-
Molecular Imaging of Very Late Antigen-4 in Acute Lung Injury.J Nucl Med. 2021 Feb;62(2):280-286. doi: 10.2967/jnumed.120.242347. Epub 2020 Jul 17. J Nucl Med. 2021. PMID: 32680928 Free PMC article.
-
PET Imaging of VLA-4 in a New BRAFV600E Mouse Model of Melanoma.Mol Imaging Biol. 2022 Jun;24(3):425-433. doi: 10.1007/s11307-021-01666-1. Epub 2021 Oct 25. Mol Imaging Biol. 2022. PMID: 34694528 Free PMC article.
-
Ex Vivo and In Vivo Evaluation of Overexpressed VLA-4 in Multiple Myeloma Using LLP2A Imaging Agents.J Nucl Med. 2016 Apr;57(4):640-5. doi: 10.2967/jnumed.115.164624. Epub 2016 Jan 7. J Nucl Med. 2016. PMID: 26742713 Free PMC article.
-
PET imaging of very late antigen-4 in melanoma: comparison of 68Ga- and 64Cu-labeled NODAGA and CB-TE1A1P-LLP2A conjugates.J Nucl Med. 2014 Nov;55(11):1856-63. doi: 10.2967/jnumed.114.144881. Epub 2014 Sep 25. J Nucl Med. 2014. PMID: 25256059 Free PMC article.
-
Use of human amniotic epithelial cells in mouse models of bleomycin-induced lung fibrosis: A systematic review and meta-analysis.PLoS One. 2018 May 17;13(5):e0197658. doi: 10.1371/journal.pone.0197658. eCollection 2018. PLoS One. 2018. PMID: 29772024 Free PMC article.
Cited by
-
Molecular Imaging of Fibrosis in Benign Diseases: An Overview of the State of the Art.Pharmaceuticals (Basel). 2024 Feb 26;17(3):296. doi: 10.3390/ph17030296. Pharmaceuticals (Basel). 2024. PMID: 38543082 Free PMC article. Review.
-
Fibrotic Disease: from Signaling Pathways and Biomarkers to Molecular Imaging.Mol Imaging Biol. 2025 Aug 11. doi: 10.1007/s11307-025-02038-9. Online ahead of print. Mol Imaging Biol. 2025. PMID: 40790290 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

