Diagnostic Efficacy of Aspergillus Galactomannan Lateral Flow Assay in Patients with Hematological Malignancies: A Prospective Multicenter Study
- PMID: 37273172
- PMCID: PMC10241129
- DOI: 10.1007/s11046-023-00749-7
Diagnostic Efficacy of Aspergillus Galactomannan Lateral Flow Assay in Patients with Hematological Malignancies: A Prospective Multicenter Study
Abstract
Background: A rapid and reliable diagnostic test is needed to reduce mortality through early diagnosis of invasive aspergillosis (IA) in patients with hematological malignancies.
Objective: To evaluate the efficacy of serum and bronchoalveolar lavage (BAL) Aspergillus galactomannan lateral flow assay (GM-LFA) in IA diagnosis and determine the correlation of GM-LFA with GM enzyme immunoassay (GM-EIA) in patients with hematological malignancies.
Methods: In this prospective multicenter study, we used serum and BAL fluid samples from patients with hematological malignancies and suspected IA and performed GM-LFA and GM-EIA. According to the EORTC/MSGERC criteria, patients were grouped as proven (n = 6), probable (n = 22), possible IA (n = 55), or no IA (n = 88). The performance of serum GM-LFA at 0.5 optical density index (ODI) and area under the curve (AUC) were calculated. Spearman's correlation analysis and kappa statistics were performed to determine the agreement between the tests.
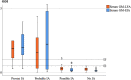
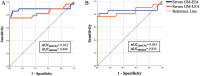
Results: GM-LFA showed an AUC of 0.832 in proven/probable IA (sensitivity [SEN], specificity [SPE], negative predictive value [NPV], and diagnostic accuracy were 75%, 100%, 92.6%, and 93.9%, respectively, at a 0.5 ODI) versus that in no IA. A moderate positive correlation was noted between the GM-LFA and GM-EIA scores (p = 0.01). The observed agreement between the tests at 0.5 ODI was almost perfect (p < 0.001). After excluding patients who received mold-active antifungal prophylaxis or treatment, the SEN, SPE, NPV, and diagnostic accuracy for proven/probable IA were 76.2%, 100%, 93.3%, and 94.5%, respectively.
Conclusions: Serum GM-LFA demonstrated high discriminatory power and good diagnostic performance for IA in patients with hematological malignancies.
Keywords: Enzyme immunoassay; Galactomannan; Hematological malignancy; Invasive aspergillosis; Lateral flow assay.
© 2023. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
All authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures

Similar articles
-
Diagnostic Utility of Galactomannan Enzyme Immunoassay in Invasive Aspergillosis in Pediatric patients with Hematological Malignancy.Mycopathologia. 2023 Dec;188(6):1055-1063. doi: 10.1007/s11046-023-00798-y. Epub 2023 Oct 9. Mycopathologia. 2023. PMID: 37806994
-
Serum Lateral Flow assay with digital reader for the diagnosis of invasive pulmonary aspergillosis: A two-centre mixed cohort study.Mycoses. 2021 Oct;64(10):1197-1202. doi: 10.1111/myc.13352. Epub 2021 Jul 24. Mycoses. 2021. PMID: 34252244 Free PMC article.
-
The Aspergillus galactomannan Ag VIRCLIA® Monotest and the sõna Aspergillus galactomannan lateral flow assay show comparable performance for the diagnosis of invasive aspergillosis.Mycoses. 2024 Aug;67(8):e13782. doi: 10.1111/myc.13782. Mycoses. 2024. PMID: 39109555
-
Utility of bronchoalveolar lavage fluid galactomannan alone or in combination with PCR for the diagnosis of invasive aspergillosis in adult hematology patients: a systematic review and meta-analysis.Crit Rev Microbiol. 2015 Feb;41(1):124-34. doi: 10.3109/1040841X.2013.804033. Epub 2013 Jun 25. Crit Rev Microbiol. 2015. PMID: 23799871
-
Galactomannan detection in broncho-alveolar lavage fluid for invasive aspergillosis in immunocompromised patients.Cochrane Database Syst Rev. 2019 May 20;5(5):CD012399. doi: 10.1002/14651858.CD012399.pub2. Cochrane Database Syst Rev. 2019. PMID: 31107543 Free PMC article.
Cited by
-
Diagnostic accuracy of galactomannan and lateral flow assay in invasive aspergillosis: A diagnostic meta-analysis.Heliyon. 2024 Jul 14;10(14):e34569. doi: 10.1016/j.heliyon.2024.e34569. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39082010 Free PMC article.
-
Evaluation of a novel aspergillus IgG lateral flow assay for the diagnosis of non-neutropenic patients with acute and subacute invasive aspergillosis.Front Cell Infect Microbiol. 2025 Jun 20;15:1599425. doi: 10.3389/fcimb.2025.1599425. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40621162 Free PMC article.
-
Diagnostic Approach to Pneumonia in Immunocompromised Hosts.J Clin Med. 2025 Jan 9;14(2):389. doi: 10.3390/jcm14020389. J Clin Med. 2025. PMID: 39860395 Free PMC article. Review.
References
-
- Heldt S, Prattes J, Eigl S, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J Infect. 2018;77(3):235–241. doi: 10.1016/j.jinf.2018.05.001. - DOI - PMC - PubMed
-
- White PL, Price JS, Posso R, Cutlan-Vaughan M, Vale L, Backx M. Evaluation of the performance of the IMMY sona Aspergillus galactomannan lateral flow assay when testing serum to aid in diagnosis of invasive aspergillosis. J Clin Microbiol. 2020;58(6):e00053–e120. doi: 10.1128/JCM.00053-20. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
