Inborn errors of human B cell development, differentiation, and function
- PMID: 37273190
- PMCID: PMC10242086
- DOI: 10.1084/jem.20221105
Inborn errors of human B cell development, differentiation, and function
Abstract
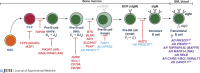
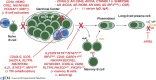
B cells develop from hematopoietic stem cells in the bone marrow. Once generated, they serve multiple roles in immune regulation and host defense. However, their most important function is producing antibodies (Ab) that efficiently clear invading pathogens. This is achieved by generating memory B cells that rapidly respond to subsequent Ag exposure, and plasma cells (PCs) that continually secrete Ab. These B cell subsets maintain humoral immunity and host protection against recurrent infections for extended periods of time. Thus, the generation of antigen (Ag)-specific memory cells and PCs underlies long-lived serological immunity, contributing to the success of most vaccines. Our understanding of immunity is often derived from animal models. However, analysis of individuals with monogenic defects that disrupt immune cell function are unprecedented models to link genotypes to clinical phenotypes, establish mechanisms of disease pathogenesis, and elucidate critical pathways for immune cell development and differentiation. Here, we review fundamental breakthroughs in unraveling the complexities of humoral immunity in humans that have come from the discovery of inborn errors disrupting B cell function.
© 2023 Tangye et al.
Conflict of interest statement
Disclosures: S.G. Tangye is a member of the Pharming Group NV Global Advisory Board for the use of leniolisib to treat individuals with inborn errors of immunity due to mutations in
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

