Tolerogenic dendritic cell reporting: Has a minimum information model made a difference?
- PMID: 37273539
- PMCID: PMC10239229
- DOI: 10.7717/peerj.15352
Tolerogenic dendritic cell reporting: Has a minimum information model made a difference?
Abstract
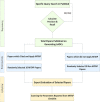
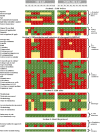
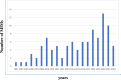
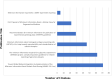
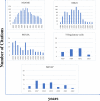
Minimum information models are reporting frameworks that describe the essential information that needs to be provided in a publication, so that the work can be repeated or compared to other work. In 2016, Minimum Information about Tolerogenic Antigen-Presenting cells (MITAP) was created to standardize the reporting on tolerogenic antigen-presenting cells, including tolerogenic dendritic cells (tolDCs). tolDCs is a generic term for dendritic cells that have the ability to (re-)establish immune tolerance; they have been developed as a cell therapy for autoimmune diseases or for the prevention of transplant rejection. Because protocols to generate these therapeutic cells vary widely, MITAP was deemed to be a pivotal reporting tool by and for the tolDC community. In this paper, we explored the impact that MITAP has had on the tolDC field. We did this by examining a subset of the available literature on tolDCs. Our analysis shows that MITAP is used in only the minority of relevant papers (14%), but where it is used the amount of metadata available is slightly increased over where it is not. From this, we conclude that MITAP has been a partial success, but that much more needs to be done if standardized reporting is to become common within the discipline.
Keywords: Cell therapy; FAIR data; Metadata sharing; Minimum information model; Open data sharing; Reporting guidelines; Standardised data reporting; Tolerogenic dendritic cell.
©2023 Sahar et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

References
-
- Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nature Genetics. 2001;29(4):365–371. doi: 10.1038/ng1201-365. - DOI - PubMed
-
- Britten CM, Janetzki S, Van der Burg SH, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJM, O’Donnell-Tormey J, Odunsi K, Old LJ, Pawelec G, Roep BO, Romero P, Hoos A, Davis MM. Minimal information about T cell assays: the process of reaching the community of T cell immunologists in cancer and beyond. Cancer Immunology, Immunotherapy. 2011;60(1):15–22. doi: 10.1007/s00262-010-0940-z. - DOI - PMC - PubMed
-
- Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, Ashburner M, Axelrod N, Baldauf S, Ballard S, Boore J, Cochrane G, Cole J, Dawyndt P, Vos PD, DePamphilis C, Edwards R, Faruque N, Feldman R, Gilbert J, Gilna P, Oliver Glöckner F, Goldstein P, Guralnick R, Haft D, Hancock D, Hermjakob H, Hertz-Fowler C, Hugenholtz P, Joint I, Kagan L, Kane M, Kennedy J, Kowalchuk G, Kottmann R, Kolker E, Kravitz S, Kyrpides N, Leebens-Mack J, Lewis SE, Li K, Lister AL, Lord P, Maltsev N, Markowitz V, Martiny J, Methe B, Mizrachi I, Moxon R, Nelson K, Parkhill J, Proctor L, White O, Sansone S-A, Spiers A, Stevens R, Swift P, Taylor C, Tateno Y, Tett A, Turner S, Ussery D, Vaughan B, Ward N, Whetzel T, Gil IS, Wilson G, Wipat A. The minimum information about a genome sequence (MIGS) specification. Nature Biotechnology. 2008;26(5):541–547. doi: 10.1038/nbt1360. - DOI - PMC - PubMed
-
- Fuchs A, Gliwiński M, Grageda N, Spiering R, Abbas AK, Appel S, Bacchetta R, Battaglia M, Berglund D, Blazar B, Bluestone JA, Bornhäuser M, Brinke AT, Brusko TM, Cools N, Cuturi MC, Geissler E, Giannoukakis N, Gołab K, Hafler DA, Van Ham SM, Hester J, Hippen K, Di Ianni M, Ilic N, Isaacs J, Issa F, Iwaszkiewicz-Grześ D, Jaeckel E, Joosten I, Klatzmann D, Koenen H, Van Kooten C, Korsgren O, Kretschmer K, Levings M, Marek-Trzonkowska NM, Martinez-Llordella M, Miljkovic D, Mills KHG, Miranda JP, Piccirillo CA, Putnam AL, Ritter T, Roncarolo MG, Sakaguchi S, Sánchez-Ramón S, Sawitzki B, Sofronic-Milosavljevic L, Sykes M, Tang Q, Vives-Pi M, Waldmann H, Witkowski P, Wood KJ, Gregori S, Hilkens CMU, Lombardi G, Lord P, Martinez-Caceres EM, Trzonkowski P. Minimum information about T regulatory cells: a step toward reproducibility and standardization. Frontiers in Immunology. 2018;8:1844. doi: 10.3389/fimmu.2017.01844. - DOI - PMC - PubMed
Further Reading
-
- Anderson AE, Swan DJ, Wong OY, Buck M, Eltherington O, Harry RA, Patterson AM, Pratt AG, Reynolds G, Doran JP, Kirby JA. Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4+ T cells partly via transforming growth factor- β 1. Clinical & Experimental Immunology. 2017;187(1):113–23. doi: 10.1111/cei.12870. - DOI - PMC - PubMed
-
- Bouchet-Delbos L, Even A, Varey E, Saïagh S, Bercegeay S, Braudeau C, Dréno B, Blancho G, Josien R, Cuturi MC, Moreau A. Preclinical assessment of autologous tolerogenic dendritic cells from end-stage renal disease patients. Transplantation. 2021;105(4):832–841. doi: 10.1097/tp.0000000000003315. - DOI - PubMed
-
- Eslami-kaliji F, Sarafbidabad M, Kiani-Esfahani A, Mirahmadi-Zare SZ, Dormiani K. 10-hydroxy-2-decenoic acid a bio-immunomodulator in tissue engineering; generates tolerogenic dendritic cells by blocking the toll-like receptor 4. Journal of Biomedical Materials Research Part A. 2021;109(9):1575–1587. doi: 10.1002/jbm.a.37152. - DOI - PubMed
-
- Funda DP, Goliáš J, Hudcovic T, Kozáková H, Špíšek R, Palová-Jelínková L. Antigen loading (eg. glutamic acid decarboxylase 65) of tolerogenic DCs (tolDCs) reduces their capacity to prevent diabetes in the non-obese diabetes (NOD)-severe combined immunodeficiency model of adoptive cotransfer of diabetes as well as in NOD mice. Frontiers in Immunology. 2018;9:290. doi: 10.3389/fimmu.2018.00290. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

