Multifunctional nanostructures: Intelligent design to overcome biological barriers
- PMID: 37273793
- PMCID: PMC10232915
- DOI: 10.1016/j.mtbio.2023.100672
Multifunctional nanostructures: Intelligent design to overcome biological barriers
Abstract
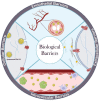
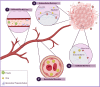
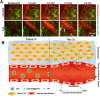
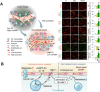
Over the past three decades, nanoscience has offered a unique solution for reducing the systemic toxicity of chemotherapy drugs and for increasing drug therapeutic efficiency. However, the poor accumulation and pharmacokinetics of nanoparticles are some of the key reasons for their slow translation into the clinic. The is intimately linked to the non-biological nature of nanoparticles and the aberrant features of solid cancer, which together significantly compromise nanoparticle delivery. New findings on the unique properties of tumors and their interactions with nanoparticles and the human body suggest that, contrary to what was long-believed, tumor features may be more mirage than miracle, as the enhanced permeability and retention based efficacy is estimated to be as low as 1%. In this review, we highlight the current barriers and available solutions to pave the way for approved nanoformulations. Furthermore, we aim to discuss the main solutions to solve inefficient drug delivery with the use of nanobioengineering of nanocarriers and the tumor environment. Finally, we will discuss the suggested strategies to overcome two or more biological barriers with one nanocarrier. The variety of design formats, applications and implications of each of these methods will also be evaluated.
Keywords: Biological barriers; Biotechnology; Cancer; Nanoparticles engineering; Nanotechnology; Targeted drug delivery.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures











References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

