Infliximab vs adalimumab: Points to consider when selecting anti-tumor necrosis factor agents in pediatric patients with Crohn's disease
- PMID: 37274072
- PMCID: PMC10237103
- DOI: 10.3748/wjg.v29.i18.2784
Infliximab vs adalimumab: Points to consider when selecting anti-tumor necrosis factor agents in pediatric patients with Crohn's disease
Abstract
Biologic agents with various mechanisms against Crohn's disease (CD) have been released and are widely used in clinical practice. However, two anti-tumor necrosis factor (TNF) agents, infliximab (IFX) and adalimumab (ADL), are the only biologic agents approved by the Food and Drug Administration for pediatric CD currently. Therefore, in pediatric CD, the choice of biologic agents should be made more carefully to achieve the therapeutic goal. There are currently no head-to-head trials of biologic agents in pediatric or adult CD. There is a lack of accumulated data for pediatric CD, which requires the extrapolation of adult data for the positioning of biologics in pediatric CD. From a pharmacokinetic point of view, IFX is more advantageous than ADL when the inflammatory burden is high, and ADL is expected to be advantageous over IFX in sustaining remission in the maintenance phase. Additionally, we reviewed the safety profile, immunogenicity, preference, and compliance between IFX and ADL and provide practical insights into the choice of anti-TNF therapy in pediatric CD. Careful evaluation of clinical indications and disease behavior is essential when prescribing anti-TNF agents. In addition, factors such as the efficacy of induction and maintenance of remission, safety profile, immunogenicity, patient preference, and compliance play an important role in evaluating and selecting treatment options.
Keywords: Adalimumab; Anti-tumor necrosis factor; Crohn’s disease; Infliximab; Pediatric.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All authors have no conflicts of interest to declare.
Figures

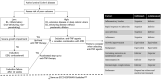
References
-
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet . 2017;390:2769–2778. - PubMed
-
- Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis . 2011;17:423–439. - PubMed
-
- US Department of Health & Human Services. Drugs @ FDA-label and approval history. In: Food and Drug Administration. [cited 2022 December 28]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti...
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

