Effectiveness of the Coronavirus Disease 2019 Bivalent Vaccine
- PMID: 37274183
- PMCID: PMC10234376
- DOI: 10.1093/ofid/ofad209
Effectiveness of the Coronavirus Disease 2019 Bivalent Vaccine
Abstract
Background: The purpose of this study was to evaluate whether a bivalent coronavirus disease 2019 (COVID-19) vaccine protects against COVID-19.
Methods: The study included employees of Cleveland Clinic in employment when the bivalent COVID-19 vaccine first became available. Cumulative incidence of COVID-19 over the following 26 weeks was examined. Protection provided by vaccination (analyzed as a time-dependent covariate) was evaluated using Cox proportional hazards regression, with change in dominant circulating lineages over time accounted for by time-dependent coefficients. The analysis was adjusted for the pandemic phase when the last prior COVID-19 episode occurred and the number of prior vaccine doses.
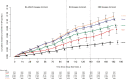
Results: Among 51 017 employees, COVID-19 occurred in 4424 (8.7%) during the study. In multivariable analysis, the bivalent-vaccinated state was associated with lower risk of COVID-19 during the BA.4/5-dominant (hazard ratio, 0.71 [95% confidence interval, .63-79]) and the BQ-dominant (0.80 [.69-.94]) phases, but decreased risk was not found during the XBB-dominant phase (0.96 [.82-.1.12]). The estimated vaccine effectiveness was 29% (95% confidence interval, 21%-37%), 20% (6%-31%), and 4% (-12% to 18%), during the BA.4/5-, BQ-, and XBB-dominant phases, respectively. The risk of COVID-19 also increased with time since the most recent prior COVID-19 episode and with the number of vaccine doses previously received.
Conclusions: The bivalent COVID-19 vaccine given to working-aged adults afforded modest protection overall against COVID-19 while the BA.4/5 lineages were the dominant circulating strains, afforded less protection when the BQ lineages were dominant, and effectiveness was not demonstrated when the XBB lineages were dominant.
Keywords: COVID-19; SARS-CoV-2; bivalent vaccine; effectiveness; vaccines.
© Crown copyright 2023.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts.
Figures


References
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. . Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397:1819–29. - PMC - PubMed
-
- Shrestha NK, Nowacki AS, Burke PC, Terpeluk P, Gordon SM. Effectiveness of mRNA COVID-19 vaccines among employees in an American healthcare system. medRxiv [Preprint: not peer reviewed]. 10 August2021. Available from: https://www.medrxiv.org/content/10.1101/2021.06.02.21258231v1. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

