Drosophila photoreceptor systems converge in arousal neurons and confer light responsive robustness
- PMID: 37274190
- PMCID: PMC10235467
- DOI: 10.3389/fnins.2023.1160353
Drosophila photoreceptor systems converge in arousal neurons and confer light responsive robustness
Abstract
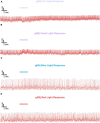
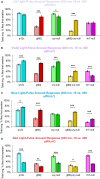
Lateral ventral neurons (LNvs) in the fly circadian neural circuit mediate behaviors other than clock resetting, including light-activated acute arousal. Converging sensory inputs often confer functional redundancy. The LNvs have three distinct light input pathways: (1) cell autonomously expressed cryptochrome (CRY), (2) rhodopsin 7 (Rh7), and (3) synaptic inputs from the eyes and other external photoreceptors that express opsins and CRY. We explored the relative photoelectrical and behavioral input contributions of these three photoreceptor systems to determine their functional impact in flies. Patch-clamp electrophysiology measuring light evoked firing frequency (FF) was performed on large LNvs (l-LNvs) in response to UV (365 nm), violet (405 nm), blue (450 nm), or red (635 nm) LED light stimulation, testing controls versus mutants that lack photoreceptor inputs gl60j, cry-null, rh7-null, and double mutant gl60j-cry-null flies. For UV, violet, and blue short wavelength light inputs, all photoreceptor mutants show significantly attenuated action potential FF responses measured in the l-LNv. In contrast, red light FF responses are only significantly attenuated in double mutant gl60j-cry-null flies. We used a light-pulse arousal assay to compare behavioral responses to UV, violet, blue and red light of control and light input mutants, measuring the awakening arousal response of flies during subjective nighttime at two different intensities to capture potential threshold differences (10 and 400 μW/cm2). The light arousal behavioral results are similar to the electrophysiological results, showing significant attenuation of behavioral light responses for mutants compared to control. These results show that the different LNv convergent photoreceptor systems are integrated and together confer functional redundancy for light evoked behavioral arousal.
Keywords: Drosophila melanogaster; cryptochrome; electrophysiology; external rhodopsin; internal rhodopsin-7; light arousal behavior; non-image forming vision; photoreceptor circuit.
Copyright © 2023 Au, Liu, Park, Nguyen, Dimalanta, Foden and Holmes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures













References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

