Little things with significant impact: miRNAs in hepatocellular carcinoma
- PMID: 37274242
- PMCID: PMC10235484
- DOI: 10.3389/fonc.2023.1191070
Little things with significant impact: miRNAs in hepatocellular carcinoma
Abstract
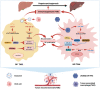
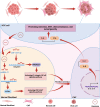
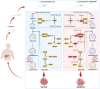
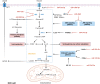
Hepatocellular carcinoma (HCC) has developed into one of the most lethal, aggressive, and malignant cancers worldwide. Although HCC treatment has improved in recent years, the incidence and lethality of HCC continue to increase yearly. Therefore, an in-depth study of the pathogenesis of HCC and the search for more reliable therapeutic targets are crucial to improving the survival quality of HCC patients. Currently, miRNAs have become one of the hotspots in life science research, which are widely present in living organisms and are non-coding RNAs involved in regulating gene expression. MiRNAs exert their biological roles by suppressing the expression of downstream genes and are engaged in various HCC-related processes, including proliferation, apoptosis, invasion, and metastasis. In addition, the expression status of miRNAs is related to the drug resistance mechanism of HCC, which has important implications for the systemic treatment of HCC. This paper reviews the regulatory role of miRNAs in the pathogenesis of HCC and the clinical applications of miRNAs in HCC in recent years.
Keywords: autophagy; ferroptosis; hepatocellular carcinoma; metabolic reprogramming; microRNA; tumor microenvironment.
Copyright © 2023 Li, Bao, Huang, Liang, Wang, Lin, Ni and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

