Blood immune cells as potential biomarkers predicting relapse-free survival of stage III/IV resected melanoma patients treated with peptide-based vaccination and interferon-alpha
- PMID: 37274275
- PMCID: PMC10233106
- DOI: 10.3389/fonc.2023.1145667
Blood immune cells as potential biomarkers predicting relapse-free survival of stage III/IV resected melanoma patients treated with peptide-based vaccination and interferon-alpha
Abstract
Introduction: Despite the recent approval of several therapies in the adjuvant setting of melanoma, tumor relapse still occurs in a significant number of completely resected stage III-IV patients. In this context, the use of cancer vaccines is still relevant and may increase the response to immune checkpoint inhibitors. We previously demonstrated safety, immunogenicity and preliminary evidence of clinical efficacy in stage III/IV resected melanoma patients subjected to a combination therapy based on peptide vaccination together with intermittent low-dose interferon-α2b, with or without dacarbazine preconditioning (https://www.clinicaltrialsregister.eu/ctr-search/search, identifier: 2008-008211-26). In this setting, we then focused on pre-treatment patient immune status to highlight possible factors associated with clinical outcome.
Methods: Multiparametric flow cytometry was used to identify baseline immune profiles in patients' peripheral blood mononuclear cells and correlation with the patient clinical outcome. Receiver operating characteristic curve, Kaplan-Meier survival and principal component analyses were used to evaluate the predictive power of the identified markers.
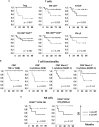
Results: We identified 12 different circulating T and NK cell subsets with significant (p ≤ 0.05) differential baseline levels in patients who later relapsed with respect to patients who remained free of disease. All 12 parameters showed a good prognostic accuracy (AUC>0.7, p ≤ 0.05) and 11 of them significantly predicted the relapse-free survival. Remarkably, 3 classifiers also predicted the overall survival. Focusing on immune cell subsets that can be analyzed through simple surface staining, three subsets were identified, namely regulatory T cells, CD56dimCD16- NK cells and central memory γδ T cells. Each subset showed an AUC>0.8 and principal component analysis significantly grouped relapsing and non-relapsing patients (p=0.034). These three subsets were used to calculate a combination score that was able to perfectly distinguish relapsing and non-relapsing patients (AUC=1; p=0). Noticeably, patients with a combined score ≥2 demonstrated a strong advantage in both relapse-free (p=0.002) and overall (p=0.011) survival as compared to patients with a score <2.
Discussion: Predictive markers may be used to guide patient selection for personalized therapies and/or improve follow-up strategies. This study provides preliminary evidence on the identification of peripheral blood immune biomarkers potentially capable of predicting the clinical response to combined vaccine-based adjuvant therapies in melanoma.
Keywords: adjuvant therapy; circulating biomarkers; combination therapy; immunotherapy; interferon alpha (IFN-α); melanoma; multiparametric flow cytometry; peptide vaccination.
Copyright © 2023 Moschella, Buccione, Ruspantini, Castiello, Rozo Gonzalez, Iacobone, Ferraresi, Palermo, Nisticò, Belardelli, Proietti, Macchia and Urbani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Clinical and Immunological Outcomes in High-Risk Resected Melanoma Patients Receiving Peptide-Based Vaccination and Interferon Alpha, With or Without Dacarbazine Preconditioning: A Phase II Study.Front Oncol. 2020 Mar 6;10:202. doi: 10.3389/fonc.2020.00202. eCollection 2020. Front Oncol. 2020. PMID: 32211314 Free PMC article.
-
CD56dim CD16- Natural Killer Cell Profiling in Melanoma Patients Receiving a Cancer Vaccine and Interferon-α.Front Immunol. 2019 Jan 29;10:14. doi: 10.3389/fimmu.2019.00014. eCollection 2019. Front Immunol. 2019. PMID: 30761123 Free PMC article.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
-
Angiogenic and immune parameters during recombinant interferon-alpha2b adjuvant treatment in patients with melanoma.Oncol Res. 2000;12(5):241-51. doi: 10.3727/096504001108747738. Oncol Res. 2000. PMID: 11417749 Clinical Trial.
-
Adjuvant therapy for resected stage III melanoma patients: high-dose interferon-alpha versus ipilimumab combined with kinases inhibitors.Tumori. 2012 Mar-Apr;98(2):185-90. doi: 10.1177/030089161209800202. Tumori. 2012. PMID: 22677983 Review.
Cited by
-
Single-cell profiling of peripheral blood mononuclear cells from patients treated with oncolytic adenovirus TILT-123 reveals baseline immune status as a predictor of therapy outcomes.Cancer Gene Ther. 2025 Jun;32(6):649-661. doi: 10.1038/s41417-025-00901-z. Epub 2025 Apr 10. Cancer Gene Ther. 2025. PMID: 40211048 Free PMC article. Clinical Trial.
-
Adjuvant anti-PD-1 therapy improves melanoma-specific survival in stage IIIC-IV melanoma patients with high tumor mutation burden and BRAF V600 mutation.Front Oncol. 2025 Aug 12;15:1618596. doi: 10.3389/fonc.2025.1618596. eCollection 2025. Front Oncol. 2025. PMID: 40874224 Free PMC article.
References
-
- Eggermont AMM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. . Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol (2015) 16:522–30. doi: 10.1016/S1470-2045(15)70122-1 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

