Hepatokines, bile acids and ketone bodies are novel Hormones regulating energy homeostasis
- PMID: 37274345
- PMCID: PMC10236950
- DOI: 10.3389/fendo.2023.1154561
Hepatokines, bile acids and ketone bodies are novel Hormones regulating energy homeostasis
Abstract
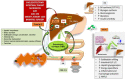
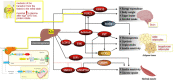
Current views show that an impaired balance partly explains the fat accumulation leading to obesity. Fetal malnutrition and early exposure to endocrine-disrupting compounds also contribute to obesity and impaired insulin secretion and/or sensitivity. The liver plays a major role in systemic glucose homeostasis through hepatokines secreted by hepatocytes. Hepatokines influence metabolism through autocrine, paracrine, and endocrine signaling and mediate the crosstalk between the liver, non-hepatic target tissues, and the brain. The liver also synthetizes bile acids (BAs) from cholesterol and secretes them into the bile. After food consumption, BAs mediate the digestion and absorption of fat-soluble vitamins and lipids in the duodenum. In recent studies, BAs act not simply as fat emulsifiers but represent endocrine molecules regulating key metabolic pathways. The liver is also the main site of the production of ketone bodies (KBs). In prolonged fasting, the brain utilizes KBs as an alternative to CHO. In the last few years, the ketogenic diet (KD) became a promising dietary intervention. Studies on subjects undergoing KD show that KBs are important mediators of inflammation and oxidative stress. The present review will focus on the role played by hepatokines, BAs, and KBs in obesity, and diabetes prevention and management and analyze the positive effects of BAs, KD, and hepatokine receptor analogs, which might justify their use as new therapeutic approaches for metabolic and aging-related diseases.
Keywords: GPBAR1; bile acids; fasting; hepatokines; ketogenic diet.
Copyright © 2023 Garruti, Baj, Cignarelli, Perrini and Giorgino.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

