Stimuli-responsive nanocarrier delivery systems for Pt-based antitumor complexes: a review
- PMID: 37274408
- PMCID: PMC10233443
- DOI: 10.1039/d3ra00866e
Stimuli-responsive nanocarrier delivery systems for Pt-based antitumor complexes: a review
Abstract
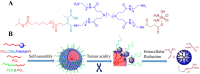
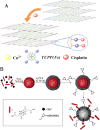
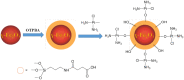
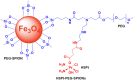
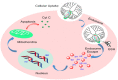
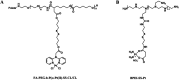
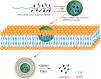
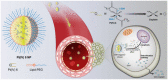
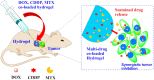
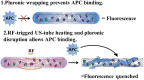
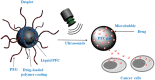
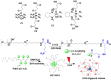
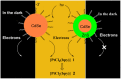
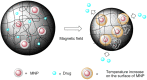
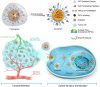
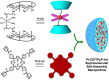
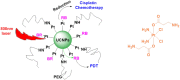
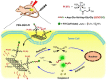
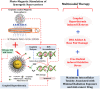
Platinum-based anticancer drugs play a crucial role in the clinical treatment of various cancers. However, the application of platinum-based drugs is heavily restricted by their severe toxicity and drug resistance/cross resistance. Various drug delivery systems have been developed to overcome these limitations of platinum-based chemotherapy. Stimuli-responsive nanocarrier drug delivery systems as one of the most promising strategies attract more attention. And huge progress in stimuli-responsive nanocarrier delivery systems of platinum-based drugs has been made. In these systems, a variety of triggers including endogenous and extracorporeal stimuli have been employed. Endogenous stimuli mainly include pH-, thermo-, enzyme- and redox-responsive nanocarriers. Extracorporeal stimuli include light-, magnetic field- and ultrasound responsive nanocarriers. In this review, we present the recent advances in stimuli-responsive drug delivery systems with different nanocarriers for improving the efficacy and reducing the side effects of platinum-based anticancer drugs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Stimuli-Responsive Gene Delivery Nanocarriers for Cancer Therapy.Nanomicro Lett. 2023 Feb 8;15(1):44. doi: 10.1007/s40820-023-01018-4. Nanomicro Lett. 2023. PMID: 36752939 Free PMC article. Review.
-
Recent Advances in Endogenous and Exogenous Stimuli-Responsive Nanocarriers for Drug Delivery and Therapeutics.Chem Pharm Bull (Tokyo). 2017;65(7):612-617. doi: 10.1248/cpb.c17-00068. Chem Pharm Bull (Tokyo). 2017. PMID: 28674332 Review.
-
Organ-restricted delivery through stimuli-responsive nanocarriers for lung cancer therapy.Life Sci. 2022 Dec 1;310:121133. doi: 10.1016/j.lfs.2022.121133. Epub 2022 Oct 26. Life Sci. 2022. PMID: 36306866 Review.
-
Recent Progress in Stimuli-Responsive Intelligent Nano Scale Drug Delivery Systems: A Special Focus Towards pH-Sensitive Systems.Curr Drug Targets. 2021;22(8):947-966. doi: 10.2174/1389450122999210128180058. Curr Drug Targets. 2021. PMID: 33511953 Review.
-
Stimuli-responsive image-guided nanocarriers as smart drug delivery platforms.Expert Opin Drug Deliv. 2022 Nov;19(11):1487-1504. doi: 10.1080/17425247.2022.2134853. Epub 2022 Oct 18. Expert Opin Drug Deliv. 2022. PMID: 36214740 Review.
Cited by
-
Recent advances in melittin-based nanoparticles for antitumor treatment: from mechanisms to targeted delivery strategies.J Nanobiotechnology. 2023 Nov 28;21(1):454. doi: 10.1186/s12951-023-02223-4. J Nanobiotechnology. 2023. PMID: 38017537 Free PMC article. Review.
-
Preparation of reversible cross-linked amphiphilic polymeric micelles with pH-responsive behavior for smart drug delivery.RSC Adv. 2023 Sep 25;13(40):28165-28178. doi: 10.1039/d3ra05575b. eCollection 2023 Sep 18. RSC Adv. 2023. PMID: 37753398 Free PMC article.
-
Exploring the Impact of Head Group Modifications on the Anticancer Activities of Fatty-Acid-like Platinum(IV) Prodrugs: A Structure-Activity Relationship Study.Int J Mol Sci. 2023 Aug 27;24(17):13301. doi: 10.3390/ijms241713301. Int J Mol Sci. 2023. PMID: 37686109 Free PMC article.
-
PEGylation Effects on Amphiphilic Platinum(IV) Complexes: Influence on Uptake, Activation, and Cytotoxicity.Pharmaceutics. 2025 Mar 29;17(4):440. doi: 10.3390/pharmaceutics17040440. Pharmaceutics. 2025. PMID: 40284435 Free PMC article.
-
pH-Responsive Block Copolymer Micelles of Temsirolimus: Preparation, Characterization and Antitumor Activity Evaluation.Int J Nanomedicine. 2024 Sep 23;19:9821-9841. doi: 10.2147/IJN.S469913. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39345910 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

