Computational Analysis of miR-140 and miR-135 as Potential Targets to Develop Combinatorial Therapeutics for Degenerative Tendinopathy
- PMID: 37274502
- PMCID: PMC10232305
- DOI: 10.4055/cios22237
Computational Analysis of miR-140 and miR-135 as Potential Targets to Develop Combinatorial Therapeutics for Degenerative Tendinopathy
Abstract
Background: Degenerative tendinopathy, a condition causing movement restriction due to high pain, highly impacts productivity and quality of life. The healing process is a complex phenomenon and involves a series of intra-cellular and inter-cellular processes. Proliferation and differentiation of the tenocyte is a major and essential process to heal degenerative tendinopathy. The recent development in microRNA (miRNA)-mediated reprogramming of the cellular function through specific pathways opened door for the development of new regenerative therapeutics. Based on information about gene expression and regulation of tendon injury and healing, we attempted to evaluate the combinatorial effect of selected miRNAs for better healing of degenerative tendinopathy.
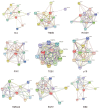
Methods: The present study was designed to evaluate the combinatorial effect of two miRNAs (has-miR-140 and has-miR-135) in the healing process of the tendon. Publicly available information/data were retrieved from appropriate platforms such as PubMed. Only molecular data, directly associated with tendinopathies, including genes/proteins and miRNAs, were used in this study. The miRNAs involved in tendinopathy were analyzed by a Bioinformatics tools (e.g., TargetScan, miRDB, and the RNA22v2). Interactive involvement of the miRNAs with key proteins involved in tendinopathy was predicted by the Insilco approach.
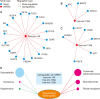
Results: Based on information available in the public domain, tendon healing-associated miRNAs were predicted to explore their therapeutic potentials. Based on computation analysis, focusing on the potential regulatory effect on tendon healing, the miR-135 and miR-140 were selected for this study. These miRNAs were found as key players in tendon healing through Rho-associated coiled-coil containing protein kinase 1 (ROCK1), IGF-1/PI3K/Akt, PIN, and Wnt signaling pathways. It was also predicted that these miRNAs may reprogram the cells to induce proliferation and differentiation activity. Many miRNAs are likely to regulate genes important for the tendinopathy healing process, and the result of this study allows an approach for miRNA-mediated regeneration of the tenocyte for tendon healing. Based on computational analysis, the role of these miRNAs in different pathways was established, and the results provided insights into the combinatorial approach of miRNA-mediated cell reprogramming.
Conclusions: In this study, the association between miRNAs and the disease was evaluated to correlate the tendinopathy genes and the relevant role of different miRNAs in their regulation. Through this study, it was established that the synergistic effect of more than one miRNA on directed reprogramming of the cell could be helpful in the regeneration of damaged tissue. It is anticipated that this study will be helpful for the design of miRNA cocktails for the orchestration of cellular reprogramming events.
Keywords: Extracellular matrix remodeling; MicroRNA; Regenerative medicine; Tendinopathy; Therapeutics.
Copyright © 2023 by The Korean Orthopaedic Association.
Conflict of interest statement
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
Figures



Similar articles
-
Comprehensive analysis of lncRNA-miRNA-mRNA networks during osteogenic differentiation of bone marrow mesenchymal stem cells.BMC Genomics. 2022 Jun 7;23(1):425. doi: 10.1186/s12864-022-08646-x. BMC Genomics. 2022. PMID: 35672672 Free PMC article.
-
Pro-inflammatory activity of long noncoding RNA FOXD2-AS1 in Achilles tendinopathy.J Orthop Surg Res. 2023 May 16;18(1):361. doi: 10.1186/s13018-023-03681-0. J Orthop Surg Res. 2023. PMID: 37194076 Free PMC article.
-
Integrated analysis of microRNA and gene expression profiles reveals a functional regulatory module associated with liver fibrosis.Gene. 2017 Dec 15;636:87-95. doi: 10.1016/j.gene.2017.09.027. Epub 2017 Sep 14. Gene. 2017. PMID: 28919164
-
The Roles of MicroRNAs in Tendon Healing and Regeneration.Front Cell Dev Biol. 2021 Jul 2;9:687117. doi: 10.3389/fcell.2021.687117. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34277629 Free PMC article. Review.
-
Moderate evidence exists for four microRNAs as potential biomarkers for tendinopathies and degenerative tendon ruptures at the upper extremity in elderly patients: conclusion of a systematic review with best-evidence synthesis.J Exp Orthop. 2023 Aug 10;10(1):81. doi: 10.1186/s40634-023-00645-5. J Exp Orthop. 2023. PMID: 37563331 Free PMC article. Review.
Cited by
-
Advancements in Therapeutic Approaches for Degenerative Tendinopathy: Evaluating Efficacy and Challenges.Int J Mol Sci. 2024 Nov 4;25(21):11846. doi: 10.3390/ijms252111846. Int J Mol Sci. 2024. PMID: 39519397 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

