Salmonella Combination Vaccines: Moving Beyond Typhoid
- PMID: 37274529
- PMCID: PMC10236507
- DOI: 10.1093/ofid/ofad041
Salmonella Combination Vaccines: Moving Beyond Typhoid
Abstract
There is now a robust pipeline of licensed and World Health Organization (WHO)-prequalified typhoid conjugate vaccines with a steady progression of national introductions. However, typhoid fever is responsible for less than half the total global burden of Salmonella disease, and even less among children aged <5 years. Invasive nontyphoidal Salmonella disease is the dominant clinical presentation of Salmonella in Africa, and over a quarter of enteric fever in Asia is due to paratyphoid A. In this article, we explore the case for combination Salmonella vaccines, review the current pipeline of these vaccines, and discuss key considerations for their development, including geographies of use, age of administration, and pathways to licensure. While a trivalent typhoid/nontyphoidal Salmonella vaccine is attractive for Africa, and a bivalent enteric fever vaccine for Asia, a quadrivalent vaccine covering the 4 main disease-causing serovars of Salmonella enterica would provide a single vaccine option for global Salmonella coverage.
Keywords: Salmonella; nontyphoidal; paratyphoid; typhoid; vaccines.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C. A. M. is an inventor of the Vi-CRM197 typhoid conjugate vaccine (US patent 2016/0263213 A1, “Typhoid Conjugate Vaccines,” current assignee: GlaxoSmithKline Biologicals SA) and the bivalent nontyphoidal Salmonella generalized modules for membrane antigens vaccine, and is a former employee of the Novartis Vaccines Institute for Global Health. All other authors report no potential conflicts.
Figures
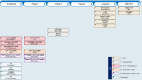

References
-
- Gavi . More typhoid conjugate vaccines, more impact. 2020. https://www.gavi.org/vaccineswork/more-typhoid-conjugate-vaccines-more-i.... Accessed 6 November 2022.
-
- The Review on Antimicrobial Resistance . Tackling drug-resistant infections globally: final report and recommendations. 2016. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20c.... Accessed 2 November 2022.
-
- Wellcome . Vaccines to tackle drug resistant infections. An evaluation of R&D opportunities. 2018. https://vaccinesforamr.org/wp-content/uploads/2018/09/Vaccines_for_AMR.pdf. Accessed 6 November 2022.
Grants and funding
LinkOut - more resources
Full Text Sources

