Prevalence, risk factors and diagnostic accuracy of non-invasive tests for NAFLD in people with type 1 diabetes
- PMID: 37274774
- PMCID: PMC10232726
- DOI: 10.1016/j.jhepr.2023.100753
Prevalence, risk factors and diagnostic accuracy of non-invasive tests for NAFLD in people with type 1 diabetes
Erratum in
-
Erratum Regarding Previously Published Articles.JHEP Rep. 2024 May 18;6(6):101097. doi: 10.1016/j.jhepr.2024.101097. eCollection 2024 Jun. JHEP Rep. 2024. PMID: 38978774 Free PMC article.
Abstract
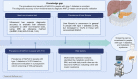
Background & aims: The epidemiology of non-alcoholic fatty liver disease (NAFLD) in people with type 1 diabetes (T1D) is not yet elucidated. This study aimed to assess the diagnostic accuracy of non-invasive tests for NAFLD, to investigate the prevalence and severity of NAFLD, and to search for factors contributing to NAFLD in people with T1D.
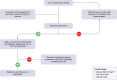
Methods: In this prospective cohort study, we consecutively screened 530 adults with T1D from a tertiary care hospital, using ultrasound (US), vibration-controlled transient elastography equipped with liver stiffness measurement (LSM) and controlled attenuation parameter, and the fatty liver index. Magnetic resonance spectroscopy (MRS) was performed in a representative subgroup of 132 individuals to validate the diagnostic accuracy of the non-invasive tests.
Results: Based on MRS as reference standard, US identified individuals with NAFLD with an AUROC of 0.98 (95% CI 0.95-1.00, sensitivity: 1.00, specificity: 0.96). The controlled attenuation parameter was also accurate with an AUROC of 0.85 (95% CI 0.77-0.93). Youden cut-off was ≥270 dB/m (sensitivity: 0.90, specificity: 0.74). The fatty liver index yielded a similar AUROC of 0.83 (95% CI 0.74-0.91), but the conventional cut-off used to rule in (≥60) had low sensitivity and specificity (0.62, 0.78). The prevalence of NAFLD in the overall cohort was 16.2% based on US. Metabolic syndrome was associated with NAFLD (OR: 2.35 [1.08-5.12], p = 0.031). The overall prevalence of LSM ≥8.0 kPa indicating significant fibrosis was 3.8%, but reached 13.2% in people with NAFLD.
Conclusions: NAFLD prevalence in individuals with T1D is 16.2%, with approximately one in 10 featuring elevated LSM. US-based screening could be considered in people with T1D and metabolic syndrome.
Impact and implications: We aimed to report on the prevalence, disease severity, and risk factors of NAFLD in type 1 diabetes (T1D), while also tackling which non-invasive test for NAFLD is the most accurate. We found that ultrasound is the best test to diagnose NAFLD. NAFLD prevalence is 16.2%, and is associated with metabolic syndrome and BMI. Elevated liver stiffness indicating fibrosis is overall not prevalent in people with T1D (3.8%), but it reaches 13.2% in those with T1D and NAFLD.
Keywords: Liver fibrosis; MRI; Metabolic syndrome; NAFLD; Transient elastography; Type 1 diabetes mellitus.
© 2023 The Author(s).
Conflict of interest statement
ED has served as a consultant for Novo Nordisk, Ely Lilly, and Boehringer Ingelheim. WK is co-inventor of a patent on the use of lipopigment imaging for disease filed by MIT/MGH. LVG declares to be member of the Advisory Board and/or Speakers Bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, and Novo Nordisk. CDB reports consulting fees and honoraria for speaking for Abbott, AstraZeneca, Boehringer-Ingelheim, A. Menarini Diagnostics, Eli Lilly, Medtronic, Novo Nordisk, and Roche, and research support from AstraZeneca, Boehringer-Ingelheim, Indigo Diabetes, and Novo Nordisk. SF has received grants from Astellas, Falk Pharma, Genfit, Gilead Sciences, GlympsBio, Janssens Pharmaceutica, Inventiva, Merck Sharp & Dome, Pfizer, and Roche. He has acted as consultant for Abbvie, Actelion, Aelin Therapeutics, Aligos Therapeutics, Allergan, Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Meyers Squibb, CSL Behring, Coherus, Echosens, Eisai, Enyo, Galapagos, Galmed, Genetech, Genfit, Gilead Sciences, Intercept, Inventiva, Janssens Pharmaceutica, Julius Clinical, Madrigal, Medimmune, Merck Sharp & Dome, NGM Bio, Novartis, Novo Nordisk, Promethera, and Roche. He has been lecturer for Abbvie, Allergan, Bayer, Eisai, Genfit, Gilead Sciences, Janssens Cilag, Intercept, Inventiva, Merck Sharp & Dome, Novo Nordisk, and Promethera. All other authors have nothing to disclose. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. - PubMed
-
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. - PubMed
-
- Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

