Clinical utility of an antibody-free LC-MS method to detect brain amyloid deposition in cognitively unimpaired individuals from the screening visit of the A4 Study
- PMID: 37274930
- PMCID: PMC10236000
- DOI: 10.1002/dad2.12451
Clinical utility of an antibody-free LC-MS method to detect brain amyloid deposition in cognitively unimpaired individuals from the screening visit of the A4 Study
Abstract
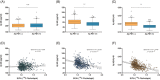
Introduction: This study explored the ability of plasma amyloid beta (Aβ)42/Aβ40 to identify brain amyloid deposition in cognitively unimpaired (CU) individuals.
Methods: Plasma Aβ was quantified with an antibody-free high-performance liquid chromatography tandem mass spectrometry method from Araclon Biotech (ABtest-MS) in a subset of 731 CU individuals from the screening visit of the Anti-Amyloid Treatment in Asymptomatic Alzheimer's (A4) Study, to assess associations of Aβ42/Aβ40 with Aβ positron emission tomography (PET).
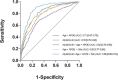
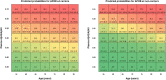
Results: A model including Aβ42/Aβ40, age, apolipoprotein E ε4, and recruitment site identified Aβ PET status with an area under the curve of 0.88 and an overall accuracy of 81%. A plasma-based pre-screening step could save up to 42% of the total number of Aβ PET scans.
Discussion: ABtest-MS accurately identified brain amyloid deposition in a population of CU individuals, supporting its implementation in AD secondary prevention trials to reduce recruitment time and costs. Although a certain degree of heterogeneity is inherent to large and multicentric trials, ABtest-MS could be more robust to pre-analytical bias compared to other immunoprecipitation mass spectrometry methods.
Highlights: Plasma amyloid beta (Aβ)42/Aβ40 accurately identified brain Aβ deposition in cognitively unimpaired individuals from the Anti-Amyloid Treatment in Asymptomatic Alzheimer's (A4) Study.The inclusion of the recruitment site in the predictive models has a non-negligible effect.A plasma biomarker-based model could reduce recruitment costs in Alzheimer's disease secondary prevention trials.Antibody-free liquid chromatography mass spectrometry methods may be more robust to pre-analytical variability than other platforms.
Keywords: Alzheimer's disease; Anti‐Amyloid Treatment in Asymptomatic Alzheimer's (A4) Study; Aβ42/Aβ40; amyloid beta; blood biomarkers; clinical trials; mass spectrometry; pre‐screening.
© 2023 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
J.A.A., M.P.L., L.S., S.C., and J.T. are full‐time employees at Araclon Biotech‐Grifols. M.E.S. is a statistical consultant at Caebi. R.A.R. and S.A.L. have no conflicts of interest relevant to this study. Author disclosures are available in the supporting information.
Figures

References
-
- Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho‐tau181 in the Alzheimer's disease neuroimaging initiative. Mol Psychiatry. 2021;26(2):429‐442. - PubMed
-
- Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66‐77. - PubMed
