Promotion of the growth and yield of Zea mays by synthetic microbial communities from Jala maize
- PMID: 37275168
- PMCID: PMC10235630
- DOI: 10.3389/fmicb.2023.1167839
Promotion of the growth and yield of Zea mays by synthetic microbial communities from Jala maize
Abstract
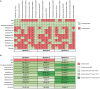
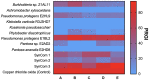
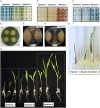
Plant growth-promoting bacteria (PGPB) are a source of nutrient supply, stimulate plant growth, and even act in the biocontrol of phytopathogens. However, these phenotypic traits have rarely been explored in culturable bacteria from native maize landraces. In this study, synthetic microbial communities (SynCom) were assembled with a set of PGPB isolated from the Jala maize landrace, some of them with additional abilities for the biocontrol of phytopathogenic fungi and the stimulation of plant-induced systemic resistance (ISR). Three SynCom were designed considering the phenotypic traits of bacterial strains, including Achromobacter xylosoxidans Z2K8, Burkholderia sp. Z1AL11, Klebsiella variicola R3J3HD7, Kosakonia pseudosacchari Z2WD1, Pantoea ananatis E2HD8, Pantoea sp. E2AD2, Phytobacter diazotrophicus Z2WL1, Pseudomonas protegens E1BL2, and P. protegens E2HL9. Plant growth promotion in gnotobiotic and greenhouse seedlings assays was performed with Conejo landrace; meanwhile, open field tests were carried out on hybrid CPL9105W maize. In all experimental models, a significant promotion of plant growth was observed. In gnotobiotic assays, the roots and shoot length of the maize seedlings increased 4.2 and 3.0 times, respectively, compared to the untreated control. Similarly, the sizes and weights of the roots and shoots of the plants increased significantly in the greenhouse assays. In the open field assay performed with hybrid CPL9105W maize, the yield increased from 11 tons/ha for the control to 16 tons/ha inoculated with SynCom 3. In addition, the incidence of rust fungal infections decreased significantly from 12.5% in the control to 8% in the treatment with SynCom 3. All SynCom designs promoted the growth of maize in all assays. However, SynCom 3 formulated with A. xylosoxidans Z2K8, Burkholderia sp. Z1AL11, K. variicola R3J3HD7, P. ananatis E2HD8, P. diazotrophicus Z2WL1, and P. protegens E1BL2 displayed the best results for promoting plant growth, their yield, and the inhibition of fungal rust. This study demonstrated the biotechnological eco-friendly plant growth-promoting potential of SynCom assemblies with culturable bacteria from native maize landraces for more sustainable and economic agriculture.
Keywords: Jala maize; biocontrol; endophytic bacteria; induced systemic resistance (ISR); plant growth-promoting bacteria (PGPB); plant-microbe interaction; synthetic microbial communities (SynCom).
Copyright © 2023 De la Vega-Camarillo, Sotelo-Aguilar, Rios-Galicia, Mercado-Flores, Arteaga-Garibay, Villa-Tanaca and Hernández-Rodríguez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

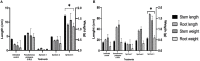

References
-
- Babu S., Prasanna R., Bidyarani N., Nain L., Shivay Y. S. (2015). Synergistic action of PGP agents and Rhizobium spp. for improved plant growth, nutrient mobilization and yields in different leguminous crops. ISBAB 4, 456–464. 10.1016/j.bcab.2015.09.004 - DOI
-
- Bashan Y. (1998). Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 16, 729–770. 10.1016/S0734-9750(98)00003-2 - DOI
LinkOut - more resources
Full Text Sources

