Evaluation of four commercial ELISAs to measure tissue factor in human plasma
- PMID: 37275179
- PMCID: PMC10233285
- DOI: 10.1016/j.rpth.2023.100133
Evaluation of four commercial ELISAs to measure tissue factor in human plasma
Abstract
Background: Under pathological conditions, tissue factor (TF)-positive extracellular vesicles (EVs) are released into the circulation and activate coagulation. Therefore, it is important to identify methods that accurately quantitate levels of TF in plasma. Enzyme-linked immunosorbent assays (ELISAs) are a fast and simple method to quantitate levels of proteins. However, there are several specific challenges with measuring TF antigen in plasma including its low concentration and the complexity of plasma.
Objectives: We aimed to evaluate the ability of 4 commercial ELISAs to measure TF in human plasma.
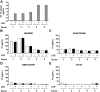
Methods: We determined the ability of 4 commercial ELISAs (Imubind, Quantikine, Human SimpleStep, and CD142 Human) to detect recombinant human TF (Innovin) (12.5-100 pg/mL), TF-positive EVs isolated from the culture supernatant from a human pancreatic cancer cell line (57 pg/mL), TF in plasma containing low levels of EV TF activity (1.2-2.6 pg/mL) from lipopolysaccharide-stimulated whole blood, and plasma containing high levels of EV TF activity (151-696 pg/mL) from patients with acute leukemia.
Results: The CD142 Human ELISA could not detect recombinant TF. Imubind and Quantikine but not Human SimpleStep detected recombinant TF spiked into plasma and TF-positive EVs isolated from the culture supernatant of a human pancreatic cancer cell line. Quantikine and Imubind could not detect low levels of TF in plasma from lipopolysaccharide-stimulated whole blood. However, Quantikine but not Imubind detected TF in plasma from acute leukemia patients with high levels of EV TF activity.
Conclusion: Our results indicate that commercial ELISAs have different abilities to detect TF. Quantikine and Imubind could not detect low levels of TF in plasma, but Quantikine detected TF in plasma with high levels of TF.
Keywords: acute leukemia; coagulation; enzyme-linked immunosorbent assay; extracellular vesicles; plasma; tissue factor.
© 2023 The Author(s).
Figures






References
-
- Grover S.P., Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–725. - PubMed
-
- Basavaraj M.G., Olsen J.O., Østerud B., Hansen J.-B. Differential ability of tissue factor antibody clones on detection of tissue factor in blood cells and microparticles. Thromb Res. 2012;130:538–546. - PubMed
-
- Yu J.L., Rak J.W. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost. 2004;2:2065–2067. - PubMed
-
- Key N., Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Sem Thromb Hemost. 2010;36:865–875. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

