Biological sex differences in renin angiotensin system enzymes ACE and ACE2 regulate normal tissue response to radiation injury
- PMID: 37275232
- PMCID: PMC10235526
- DOI: 10.3389/fphys.2023.1191237
Biological sex differences in renin angiotensin system enzymes ACE and ACE2 regulate normal tissue response to radiation injury
Abstract
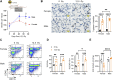
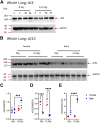
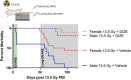
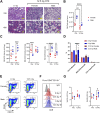
Introduction: In experimental animal models, biological sex-differences in the manifestation and severity of normal tissue radiation injury have been well-documented. Previously we demonstrated male and female rats have differential and highly reproducible responses to high-dose partial body irradiation (PBI) with male rats having greater susceptibility to both gastrointestinal acute radiation syndrome (GI-ARS) and radiation pneumonitis than female rats. Methods: In the current study, we have investigated whether differential expression of the renin-angiotensin system (RAS) enzymes angiotensin converting enzyme (ACE) and ACE2 contribute to the observed sex-related differences in radiation response. Results: During the period of symptomatic pneumonitis, the relative ratio of ACE to ACE2 (ACE/ACE2) protein in the whole lung was significantly increased by radiation in male rats alone. Systemic treatment with small molecule ACE2 agonist diminazene aceturate (DIZE) increased lung ACE2 activity and reduced morbidity during radiation pneumonitis in both sexes. Notably DIZE treatment also abrogated morbidity in male rats during GI-ARS. We then evaluated the contribution of the irradiated bone marrow (BM) compartment on lung immune cell infiltration and ACE imbalance during pneumonitis. Transplantation of bone marrow from irradiated donors increased both ACE-expressing myeloid cell infiltration and immune ACE activity in the lung during pneumonitis compared to non-irradiated donors. Discussion: Together, these data demonstrate radiation induces a sex-dependent imbalance in the renin-angiotensin system enzymes ACE and ACE2. Additionally, these data suggest a role for ACE-expressing myeloid cells in the pathogenesis of radiation pneumonitis. Finally, the observed sex-differences underscore the need for consideration of sex as a biological variable in the development of medical countermeasures for radiation exposure.
Keywords: ACE; ACE2; GI-ARS; biological sex; medical countermeasures; myeloid cells; radiation pneumonitis; renin angiotensin system.
Copyright © 2023 Sharma, Frei, Fish, Gasperetti, Veley, Szalewski, Nissen and Himburg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Barhoumi T., Alghanem B., Shaibah H., Mansour F. A., Alamri H. S., Akiel M. A., et al. (2021). SARS-CoV-2 coronavirus spike protein-induced apoptosis, inflammatory, and oxidative stress responses in THP-1-like-macrophages: Potential role of angiotensin-converting enzyme inhibitor (perindopril). Front. Immunol. 12, 728896. 10.3389/fimmu.2021.728896 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

