What a difference timing makes: Cortisol effects on neural underpinnings of emotion regulation
- PMID: 37275340
- PMCID: PMC10239016
- DOI: 10.1016/j.ynstr.2023.100544
What a difference timing makes: Cortisol effects on neural underpinnings of emotion regulation
Abstract
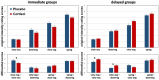
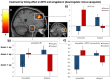
The ability of emotion regulation under stress is of crucial importance to psychosocial health. Yet, the dynamic function of stress hormones for the cognitive control of emotions over time via non-genomic and genomic cortisol effects remains to be elucidated. In this randomized, double-blind, placebo-controlled neuroimaging experiment, 105 participants (54 men, 51 women) received 20 mg hydrocortisone (cortisol) or a placebo either 30min (rapid, non-genomic cortisol effects) or 90min (slow, genomic cortisol effects) prior to a cognitive reappraisal task including different regulatory goals (i.e., downregulate vs. upregulate negative emotions). On the behavioral level, cortisol rapidly reduced and slowly enhanced emotional responsivity to negative pictures. However, only slow cortisol effects improved downregulation of negative emotions. On the neural level, cortisol rapidly enhanced, but slowly reduced amygdala and dorsolateral prefrontal activation as well as functional connectivity between both structures in the down- minus upregulate contrast. This interaction speaks for an effortful but ineffective regulation of negative emotions during rapid cortisol effects and improved emotion regulation capacities during slow cortisol effects. Taken together, these results indicate a functional shift of cortisol effects on emotion regulation processes over time which may foster successful adaptation to and recovery from stressful life events.
Keywords: Cognitive reappraisal; Functional magnetic resonance imaging; Genomic cortisol effects; Glucocorticoids; Stress hormones.
© 2023 The Authors.
Conflict of interest statement
None.
Figures

Similar articles
-
Cortisol promotes the cognitive regulation of high intensive emotions independent of timing.Eur J Neurosci. 2022 May;55(9-10):2684-2698. doi: 10.1111/ejn.15182. Epub 2021 Mar 27. Eur J Neurosci. 2022. PMID: 33709613 Clinical Trial.
-
Restoring emotional stability: Cortisol effects on the neural network of cognitive emotion regulation.Behav Brain Res. 2019 Nov 18;374:111880. doi: 10.1016/j.bbr.2019.03.049. Epub 2019 Apr 1. Behav Brain Res. 2019. PMID: 30946860
-
Neural circuitry of emotion regulation: Effects of appraisal, attention, and cortisol administration.Cogn Affect Behav Neurosci. 2017 Apr;17(2):437-451. doi: 10.3758/s13415-016-0489-1. Cogn Affect Behav Neurosci. 2017. PMID: 28032303 Clinical Trial.
-
Delayed effects of acute stress on cognitive emotion regulation.Psychoneuroendocrinology. 2021 Mar;125:105101. doi: 10.1016/j.psyneuen.2020.105101. Epub 2020 Dec 5. Psychoneuroendocrinology. 2021. PMID: 33460986
-
Change in neural response during emotion regulation is associated with symptom reduction in cognitive behavioral therapy for anxiety disorders.J Affect Disord. 2020 Jun 15;271:207-214. doi: 10.1016/j.jad.2020.04.001. Epub 2020 Apr 13. J Affect Disord. 2020. PMID: 32479318 Free PMC article. Review.
Cited by
-
Mixed-methods analysis of sickness behavior during a natural experiment: An integrative single-case study.Compr Psychoneuroendocrinol. 2025 May 22;23:100301. doi: 10.1016/j.cpnec.2025.100301. eCollection 2025 Aug. Compr Psychoneuroendocrinol. 2025. PMID: 40520414 Free PMC article.
References
LinkOut - more resources
Full Text Sources

