Staphylococcus aureus δ-toxin present on skin promotes the development of food allergy in a murine model
- PMID: 37275864
- PMCID: PMC10235538
- DOI: 10.3389/fimmu.2023.1173069
Staphylococcus aureus δ-toxin present on skin promotes the development of food allergy in a murine model
Abstract
Background: Patients with food allergy often suffer from atopic dermatitis, in which Staphylococcus aureus colonization is frequently observed. Staphylococcus aureus δ-toxin activates mast cells and promotes T helper 2 type skin inflammation in the tape-stripped murine skin. However, the physiological effects of δ-toxin present on the steady-state skin remain unknown. We aimed to investigate whether δ-toxin present on the steady-state skin impacts the development of food allergy.
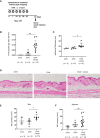
Material and methods: The non-tape-stripped skins of wild-type, KitW-sh/W-sh, or ST2-deficient mice were treated with ovalbumin (OVA) with or without δ-toxin before intragastric administration of OVA. The frequency of diarrhea, numbers of jejunum or skin mast cells, and serum levels of OVA-specific IgE were measured. Conventional dendritic cell 2 (cDC2) in skin and lymph nodes (LN) were analyzed. The cytokine levels in the skin tissues or culture supernatants of δ-toxin-stimulated murine keratinocytes were measured. Anti-IL-1α antibody-pretreated mice were analyzed.
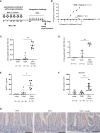
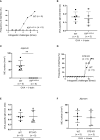
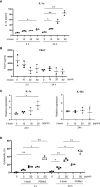
Results: Stimulation with δ-toxin induced the release of IL-1α, but not IL-33, in murine keratinocytes. Epicutaneous treatment with OVA and δ-toxin induced the local production of IL-1α. This treatment induced the translocation of OVA-loaded cDC2 from skin to draining LN and OVA-specific IgE production, independently of mast cells and ST2. This resulted in OVA-administered food allergic responses. In these models, pretreatment with anti-IL-1α antibody inhibited the cDC2 activation and OVA-specific IgE production, thereby dampening food allergic responses.
Conclusion: Even without tape stripping, δ-toxin present on skin enhances epicutaneous sensitization to food allergen in an IL-1α-dependent manner, thereby promoting the development of food allergy.
Keywords: IL-1α; IgE; Staphylococcus aureus δ-toxin; epicutaneous sensitization; food allergy; murine model.
Copyright © 2023 Yamada, Kaitani, Izawa, Ando, Kamei, Uchida, Maehara, Kojima, Yamamoto, Wang, Nagamine, Maeda, Uchida, Nakano, Ohtsuka, Ogawa, Okumura, Shimizu and Kitaura.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Thymic stromal lymphopoietin rather than IL-33 drives food allergy after epicutaneous sensitization to food allergen.J Allergy Clin Immunol. 2023 Jun;151(6):1660-1666.e4. doi: 10.1016/j.jaci.2023.02.025. Epub 2023 Mar 4. J Allergy Clin Immunol. 2023. PMID: 36878383 Free PMC article.
-
IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells.J Allergy Clin Immunol. 2016 Nov;138(5):1356-1366. doi: 10.1016/j.jaci.2016.03.056. Epub 2016 Jun 2. J Allergy Clin Immunol. 2016. PMID: 27372570 Free PMC article.
-
The optimal age for epicutaneous sensitization following tape-stripping in BALB/c mice.Allergol Int. 2018 Jul;67(3):380-387. doi: 10.1016/j.alit.2018.01.003. Epub 2018 Feb 10. Allergol Int. 2018. PMID: 29439856
-
Staphylococcus δ-toxin induces allergic skin disease by activating mast cells.Nature. 2013 Nov 21;503(7476):397-401. doi: 10.1038/nature12655. Epub 2013 Oct 30. Nature. 2013. PMID: 24172897 Free PMC article.
-
Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis.J Allergy Clin Immunol. 2013 Feb;131(2):451-60.e1-6. doi: 10.1016/j.jaci.2012.11.032. J Allergy Clin Immunol. 2013. PMID: 23374269 Free PMC article.
Cited by
-
Industrial Trans Fatty Acids Promote the Development of Food Allergy in a Mouse Model.Allergy Asthma Immunol Res. 2025 Mar;17(2):252-270. doi: 10.4168/aair.2025.17.2.252. Allergy Asthma Immunol Res. 2025. PMID: 40204509 Free PMC article.
-
The Role of Skin Dysbiosis and Quorum Sensing in Atopic Dermatitis.JID Innov. 2025 May 4;5(4):100377. doi: 10.1016/j.xjidi.2025.100377. eCollection 2025 Jul. JID Innov. 2025. PMID: 40510906 Free PMC article. Review.
-
Skin Predictive Biomarkers for the Development of Atopic Dermatitis and Food Allergy in Infants.Allergy Asthma Immunol Res. 2024 Jul;16(4):323-337. doi: 10.4168/aair.2024.16.4.323. Allergy Asthma Immunol Res. 2024. PMID: 39155734 Free PMC article. Review.
-
Staphylococcus aureus in Inflammation and Pain: Update on Pathologic Mechanisms.Pathogens. 2025 Feb 12;14(2):185. doi: 10.3390/pathogens14020185. Pathogens. 2025. PMID: 40005560 Free PMC article. Review.
-
A Mixture of Four Probiotic Strains (Probiatop®) Mitigates Food Allergy to Ovalbumin in Mice.Probiotics Antimicrob Proteins. 2024 Oct 15. doi: 10.1007/s12602-024-10386-1. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 39405021
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

