The role of tumor-infiltrating lymphocytes in triple-negative breast cancer and the research progress of adoptive cell therapy
- PMID: 37275874
- PMCID: PMC10233026
- DOI: 10.3389/fimmu.2023.1194020
The role of tumor-infiltrating lymphocytes in triple-negative breast cancer and the research progress of adoptive cell therapy
Abstract
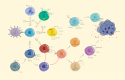
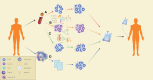
The treatment outcome of breast cancer is closely related to estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression. Triple-negative breast cancer (TNBC) lacking ER, PR, and HER2 expression has limited treatment options and a poor prognosis. Tumor-infiltrating lymphocytes (TILs) play a role in promoting or resisting tumors by affecting the tumor microenvironment and are known as key regulators in breast cancer progression. However, treatments for TNBC (e.g., surgery, chemotherapy and radiotherapy) have non-satisfaction's curative effect so far. This article reviews the role of different types of TILs in TNBC and the research progress of adoptive cell therapy, aiming to provide new therapeutic approaches for TNBC.
Keywords: adoptive cell therapy (ACT); breast cancer; solid tumor; triple-negative breast cancer (TNBC); tumor-infiltrating lymphocytes (TILs).
Copyright © 2023 Li and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study.Hum Pathol. 2013 Oct;44(10):2055-63. doi: 10.1016/j.humpath.2013.03.010. Epub 2013 May 21. Hum Pathol. 2013. PMID: 23701942 Free PMC article.
-
Tumor-infiltrating lymphocytes are significantly associated with better overall survival and disease-free survival in triple-negative but not estrogen receptor-positive breast cancers.Hum Pathol. 2017 Jun;64:7-12. doi: 10.1016/j.humpath.2017.01.004. Epub 2017 Jan 30. Hum Pathol. 2017. PMID: 28153508
-
Immunological therapy: A novel thriving area for triple-negative breast cancer treatment.Cancer Lett. 2019 Feb 1;442:409-428. doi: 10.1016/j.canlet.2018.10.042. Epub 2018 Nov 9. Cancer Lett. 2019. PMID: 30419345 Review.
-
Augmentation of antitumor function of tumor-infiltrating lymphocytes against triple-negative breast cancer by PD-1 blockade.Cell Biol Int. 2022 Feb;46(2):278-287. doi: 10.1002/cbin.11729. Epub 2021 Dec 9. Cell Biol Int. 2022. PMID: 34854515
-
Therapeutic Potential of Tumor Metabolic Reprogramming in Triple-Negative Breast Cancer.Int J Mol Sci. 2023 Apr 8;24(8):6945. doi: 10.3390/ijms24086945. Int J Mol Sci. 2023. PMID: 37108109 Free PMC article. Review.
Cited by
-
Communicator Extraordinaire: Extracellular Vesicles in the Tumor Microenvironment Are Essential Local and Long-Distance Mediators of Cancer Metastasis.Biomedicines. 2023 Sep 14;11(9):2534. doi: 10.3390/biomedicines11092534. Biomedicines. 2023. PMID: 37760975 Free PMC article. Review.
-
Implications of tumor-infiltrating lymphocytes in early-stage triple-negative breast cancer: clinical oncologist perspectives.Transl Breast Cancer Res. 2023 Oct 23;5:4. doi: 10.21037/tbcr-23-43. eCollection 2024. Transl Breast Cancer Res. 2023. PMID: 38751669 Free PMC article. Review.
-
ASO Author Reflections: Sequence of Treatment in Clinically Node-Negative T1 Triple-Negative Breast Cancer.Ann Surg Oncol. 2023 Dec;30(13):8455-8456. doi: 10.1245/s10434-023-14122-x. Epub 2023 Aug 11. Ann Surg Oncol. 2023. PMID: 37566286 No abstract available.
-
Current Status of Breast Cancer Immunotherapy and Prognosis-Related Markers.Breast Cancer (Dove Med Press). 2025 Apr 15;17:339-348. doi: 10.2147/BCTT.S506949. eCollection 2025. Breast Cancer (Dove Med Press). 2025. PMID: 40256248 Free PMC article. Review.
-
Recent Advances in Immunotherapy and Targeted Therapy of Triple Negative Breast Cancer.Curr Pharm Biotechnol. 2025;26(3):365-391. doi: 10.2174/0113892010303244240718075729. Curr Pharm Biotechnol. 2025. PMID: 39092645 Review.
References
-
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. . Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the st. gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol (2011) 22(8):1736–47. doi: 10.1093/annonc/mdr304 - DOI - PMC - PubMed
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer (2007) 109(9):1721–8. doi: 10.1002/cncr.22618 - DOI - PubMed
-
- Dieci MV, Radosevic-Robin N, Fineberg S, van den Eynden G, Ternes N, Penault-Llorca F, et al. . Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the international immuno-oncology biomarker working group on breast cancer. Semin Cancer Biol (2018) 52(Pt 2):16–25. doi: 10.1016/j.semcancer.2017.10.003 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

